Abstract
Purpose
Neoadjuvant chemotherapy (NAC) followed by surgery is the standard treatment for advanced esophageal squamous cell carcinoma (ESCC) in Japan. Computed tomography (CT) is usually used to assess the therapeutic effect of NAC; however, there are no reliable criteria for predicting pathological response or patient prognosis.
Methods
We included 84 patients who underwent esophagectomy between January 2009 and December 2018 and retrospectively reviewed their CT scans performed before and after NAC. The reduction rate of the largest tumor area (TA), long diameter (LD), and short diameter (SD) were measured on a transverse CT image. The pathological response and cutoff values were calculated using the receiver operating characteristic curve, and the most suitable ones for determining the effect were examined.
Results
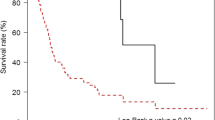
The areas under the curve for predicting responders to NAC based on the reduction rate of the TA, LD, and SD were 0.755, 0.761, and 0.781, respectively. The optimal cutoff value of the SD reduction rate for predicting responders to NAC was 22%. An SD reduction ≥ 22% was an independent prognostic factor for overall survival in univariate (p = 0.005, hazard ratio [HR] = 2.755) and multivariate analyses (p = 0.030 HR 2.690). Furthermore, an SD reduction of ≥ 22% was also an independent prognostic factor for relapse-free survival in the univariate (p = 0.007, HR = 2.491) and multivariate analyses (p = 0.007, HR = 0.030).
Conclusions
The reduction rate of the tumor SD is a simple predictor of pathological response and patient prognosis.




Similar content being viewed by others
References
Rice TW, Rusch VW, Apperson-Hansen C, Allen MS, Chen LQ, Hunter JG, Kesler KA, Law S, Lerut TE, Reed CE, Salo JA, Scott WJ, Swisher SG, Watson TJ, Blackstone EH (2009) Worldwide esophageal cancer collaboration. Dis Esophagus 22(1):1–8. https://doi.org/10.1111/j.1442-2050.2008.00901.x
Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, Ikeda K, Kanda T, Tsujinaka T, Nakamura K, Fukuda H (2012) A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 19(1):68–74. https://doi.org/10.1245/s10434-011-2049-9
Griffith JF, Chan AC, Chow LT, Leung SF, Lam YH, Liang EY, Chung SC, Metreweli C (1999) Assessing chemotherapy response of squamous cell oesophageal carcinoma with spiral CT. Br J Radiol 72(859):678–684. https://doi.org/10.1259/bjr.72.859.10624325
Jones DR, Parker LA Jr, Detterbeck FC, Egan TM (1999) Inadequacy of computed tomography in assessing patients with esophageal carcinoma after induction chemoradiotherapy. Cancer 85(5):1026–1032. https://doi.org/10.1002/(sici)1097-0142(19990301)85:5%3c1026::aid-cncr3%3e3.0.co;2-n
Zhang XY, Yan WP, Sun Y, Li XT, Chen Y, Fan MY, Wu Y, Liang Z, Xiong HC, Wang ZL, Sun YS, Chen KN (2015) CT signs can predict treatment response and long-term survival: a study in locally advanced esophageal cancer with preoperative chemotherapy. Ann Surg Oncol 22(Suppl 3):S1380-1387. https://doi.org/10.1245/s10434-015-4531-2
Westerterp M, van Westreenen HL, Reitsma JB, Hoekstra OS, Stoker J, Fockens P, Jager PL, Van Eck-Smit BL, Plukker JT, van Lanschot JJ, Sloof GW (2005) Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy–systematic review. Radiology 236(3):841–851. https://doi.org/10.1148/radiol.2363041042
Molena D, Sun HH, Badr AS, Mungo B, Sarkaria IS, Adusumilli PS, Bains MS, Rusch VW, Ilson DH, Rizk NP (2014) Clinical tools do not predict pathological complete response in patients with esophageal squamous cell cancer treated with definitive chemoradiotherapy. Dis Esophagus 27(4):355–359. https://doi.org/10.1111/dote.12126
Hatogai K, Fujii S, Kojima T, Daiko H, Kadota T, Fujita T, Yoshino T, Doi T, Takiguchi Y, Ohtsu A (2016) Prognostic significance of tumor regression grade for patients with esophageal squamous cell carcinoma after neoadjuvant chemotherapy followed by surgery. J Surg Oncol 113(4):390–396. https://doi.org/10.1002/jso.24151
Brierley J, Gospodarowicz M, Wittekind C (2017) TNM classification of malignant tumours, 8th edn. Wiley, New York
Wakatsuki K, Matsumoto S, Migita K, Ito M, Kunishige T, Nakade H, Nakatani M, Kitano M, Takano M, Obayashi C, Sho M (2017) Usefulness of computed tomography density of a tumor in predicting the response of advanced esophageal cancer to preoperative chemotherapy. Surgery 162(4):823–835. https://doi.org/10.1016/j.surg.2017.06.003
Hara H, Tahara M, Daiko H, Kato K, Igaki H, Kadowaki S, Tanaka Y, Hamamoto Y, Matsushita H, Nagase M, Hosoya Y (2013) Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci 104(11):1455–1460. https://doi.org/10.1111/cas.12274
Kitagawa Y, Uno T, Oyama T, Kato K, Kato H, Kawakubo H, Kawamura O, Kusano M, Kuwano H, Takeuchi H, Toh Y, Doki Y, Naomoto Y, Nemoto K, Booka E, Matsubara H, Miyazaki T, Muto M, Yanagisawa A, Yoshida M (2019) Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus 16(1):1–24. https://doi.org/10.1007/s10388-018-0641-9
Japan Esophageal S (2017) Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus 14(1):1–36. https://doi.org/10.1007/s10388-016-0551-7
Li SH, Rau KM, Lu HI, Wang YM, Tien WY, Liang JL, Lin WC (2012) Pre-treatment maximal oesophageal wall thickness is independently associated with response to chemoradiotherapy in patients with T3–4 oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg 42(6):958–964. https://doi.org/10.1093/ejcts/ezs136
Beer AJ, Wieder HA, Lordick F, Ott K, Fischer M, Becker K, Stollfuss J, Rummeny EJ (2006) Adenocarcinomas of esophagogastric junction: multi-detector row CT to evaluate early response to neoadjuvant chemotherapy. Radiology 239(2):472–480. https://doi.org/10.1148/radiol.2391050043
Swisher SG, Maish M, Erasmus JJ, Correa AM, Ajani JA, Bresalier R, Komaki R, Macapinlac H, Munden RF, Putnam JB, Rice D, Smythe WR, Vaporciyan AA, Walsh GL, Wu TT, Roth JA (2004) Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg 78(4):1152–1160; discussion 1152–1160. https://doi.org/10.1016/j.athoracsur.2004.04.046
van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, SpillenaarBilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A, Group C (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366(22):2074–2084. https://doi.org/10.1056/NEJMoa1112088
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Deng HY, Wang WP, Wang YC, Hu WP, Ni PZ, Lin YD, Chen LQ (2017) Neoadjuvant chemoradiotherapy or chemotherapy? A comprehensive systematic review and meta-analysis of the options for neoadjuvant therapy for treating oesophageal cancer. Eur J Cardiothorac Surg 51(3):421–431. https://doi.org/10.1093/ejcts/ezw315
Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, Mao W, Xiang J, Han Y, Chen Z, Yang H, Wang J, Pang Q, Zheng X, Yang H, Li T, Lordick F, D’Journo XB, Cerfolio RJ, Korst RJ, Novoa NM, Swanson SJ, Brunelli A, Ismail M, Fernando HC, Zhang X, Li Q, Wang G, Chen B, Mao T, Kong M, Guo X, Lin T, Liu M, Fu J, Group AMETSC (2018) Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol 36(27):2796–2803. https://doi.org/10.1200/JCO.2018.79.1483
Yokota T, Igaki H, Kato K, Tsubosa Y, Mizusawa J, Katayama H, Nakamura K, Fukuda H, Kitagawa Y (2016) Accuracy of preoperative diagnosis of lymph node metastasis for thoracic esophageal cancer patients from JCOG9907 trial. Int J Clin Oncol 21(2):283–288. https://doi.org/10.1007/s10147-015-0899-z
Konieczny A, Meyer P, Schnider A, Komminoth P, Schmid M, Lombriser N, Weishaupt D (2013) Accuracy of multidetector-row CT for restaging after neoadjuvant treatment in patients with oesophageal cancer. Eur Radiol 23(9):2492–2502. https://doi.org/10.1007/s00330-013-2844-8
Lordick F, Ott K, Krause BJ, Weber WA, Becker K, Stein HJ, Lorenzen S, Schuster T, Wieder H, Herrmann K, Bredenkamp R, Hofler H, Fink U, Peschel C, Schwaiger M, Siewert JR (2007) PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol 8(9):797–805. https://doi.org/10.1016/S1470-2045(07)70244-9
Makino T, Doki Y, Miyata H, Yasuda T, Yamasaki M, Fujiwara Y, Takiguchi S, Higuchi I, Hatazawa J, Monden M (2008) Use of (18)F-fluorodeoxyglucose-positron emission tomography to evaluate responses to neo-adjuvant chemotherapy for primary tumor and lymph node metastasis in esophageal squamous cell carcinoma. Surgery 144(5):793–802. https://doi.org/10.1016/j.surg.2008.06.026
Djuric-Stefanovic A, Micev M, Stojanovic-Rundic S, Pesko P, Saranovic D (2015) Absolute CT perfusion parameter values after the neoadjuvant chemoradiotherapy of the squamous cell esophageal carcinoma correlate with the histopathologic tumor regression grade. Eur J Radiol 84(12):2477–2484. https://doi.org/10.1016/j.ejrad.2015.09.025
Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G et al (1994) Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations Cancer 73(11):2680–2686. https://doi.org/10.1002/1097-0142(19940601)73:11%3c2680::aid-cncr2820731105%3e3.0.co;2-c
Nakamura K, Kato K, Igaki H, Ito Y, Mizusawa J, Ando N, Udagawa H, Tsubosa Y, Daiko H, Hironaka S, Fukuda H, Kitagawa Y, Japan Esophageal Oncology Group/Japan Clinical Oncology G (2013) Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol 43(7):752–755. https://doi.org/10.1093/jjco/hyt061
Author information
Authors and Affiliations
Contributions
Study conception and design: Matsumoto, Sho; acquisition of data: Matsumoto, Wakatsuki; analysis and interpretation of data: Matsumoto, Nakade, Miyao, Tatsumi; drafting of manuscript: Matsumoto, Kunishige, Tsujimoto; critical revision of manuscript: Matsumoto, Sho.
Corresponding author
Ethics declarations
Ethical approval
The research was performed in accordance with the 1964 Helsinki declaration and its later amendments and approved by the Research Ethics Committee of the Nara Medical University (No. 2104).
Informed consent
Informed consent was obtained from all participants included in the present study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Matsumoto, S., Wakatsuki, K., Nakade, H. et al. Impact of CT-assessed changes in tumor size after neoadjuvant chemotherapy on pathological response and survival of patients with esophageal squamous cell carcinoma. Langenbecks Arch Surg 407, 965–974 (2022). https://doi.org/10.1007/s00423-022-02430-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-022-02430-x