Abstract
Background
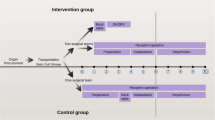
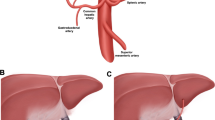
Ex vivo normothermic machine liver perfusion (NMLP) involves artificial cannulation of vessels and generation of flow pressures. This could lead to shear stress–induced endothelial damage, predisposing to vascular complications, or improved preservation of donor artery quality. This study aims to assess the spatial donor hepatic artery (HA) endothelial quality downstream of the cannulation site after end-ischaemic NMLP.
Methods
Remnant HA segments from the coeliac trunk up to the gastroduodenal artery branching were obtained after NMLP (n = 15) and after static cold storage (SCS) preservation (n = 15). Specimens were fixed in 10% neutral buffered formalin and sectioned at pre-determined anatomical sites downstream of the coeliac trunk. CD31 immunohistostaining was used to assess endothelial integrity by a 5-point ordinal scale (grade 0: intact endothelial lining, grade 5: complete denudation). Endothelial integrity after SCS was used as a control for the state of the endothelium at commencement of NMP.
Results
In the SCS specimens, regardless of the anatomical site, near complete endothelial denudation was present throughout the HA (median scores 4.5–5). After NMLP, significantly less endothelial loss in the distal HA was present compared to SCS grafts (NMLP vs. SCS: median grade 3 vs. 4.5; p = 0.042). In NMLP specimens, near complete endothelial denudation was present at the cannulation site in all cases (median grade: 5), with significantly less loss of the endothelial lining the further from the cannulation site (proximal vs. distal, median grade 5 vs. 3; p = 0.005).
Conclusion
Loss of endothelial lining throughout the HA after SCS and at the cannulation site after NMLP suggests extensive damage related to surgical handling and preservation injury. Gradual improved endothelial lining along more distal sites of the HA after NMLP indicates potential for re-endothelialisation. The regenerative effect of NMLP on artery quality seems to occur to a greater extent further from the cannulation site. Therefore, arterial cannulation for machine perfusion of liver grafts should ideally be as proximal as possible on the coeliac trunk or aortic patch, while the site of anastomosis should preferentially be attempted distal on the common HA.



Similar content being viewed by others
Abbreviations
- NMLP:
-
Normothermic machine liver perfusion
- SCS:
-
Static cold storage
- DCD:
-
Donation after cardiac death
- DBD:
-
Donation after brain death
- HA:
-
Hepatic artery
- CIT:
-
Cold ischaemia time
References
Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CD et al (2018) A randomized trial of normothermic preservation in liver transplantation. Nature 557(7703):50
Mergental H, Perera M, Laing R, Muiesan P, Isaac J, Smith A et al (2016) Transplantation of declined liver allografts following normothermic ex-situ evaluation. Am J Transplant 16(11):3235–3245
Watson C, Kosmoliaptsis V, Randle L, Russell N, Griffiths W, Davies S et al (2016) Preimplant normothermic liver perfusion of a suboptimal liver donated after circulatory death. Am J Transplant 16(1):353–357
Dewey C, Bussolari S, Gimbrone M, Davies PF (1981) The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng 103(3):177–185
Davies PF, Civelek M, Fang Y, Fleming I (2013) The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res 99(2):315–327
van der Plaats A, Leuvenink H, van Goor H, Wiersema-Buist J, Verkerke G, Rakhorst G et al (eds) (2005) Hypothermic machine perfusion of the liver and the critical balance between perfusion pressures and endothelial injury. Transplant Proc 37(1):332–334
Olschewski P, Gaß P, Ariyakhagorn V, Jasse K, Hunold G, Menzel M et al (2010) The influence of storage temperature during machine perfusion on preservation quality of marginal donor livers. Cryobiology 60(3):337–343
Yau JW, Teoh H, Verma S (2015) Endothelial cell control of thrombosis. BMC Cardiovasc Disord 15(1):130
Abbasoglu O, Levy MF, Vodapally MS, Goldstein RM, Husberg BS, Gonwa TA et al (1997) Hepatic artery stenosis after liver transplantation-incidence, presentation, treatment, and long term outcome1. Transplantation 63(2):250–255
Bekker J, Ploem S, De Jong K (2009) Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant 9(4):746–757
Mourad MM, Liossis C, Gunson BK, Mergental H, Isaac J, Muiesan P et al (2014) Etiology and management of hepatic artery thrombosis after adult liver transplantation. Liver Transpl 20(6):713–723
Oh C-K, Pelletier SJ, Sawyer RG, Dacus AR, McCullough CS, Pruett TL et al (2001) Uni-and multi-variate analysis of risk factors for early and late hepatic artery thrombosis after liver transplantation. Transplantation 71(6):767–772
Silva MA, Jambulingam PS, Gunson BK, Mayer D, Buckels JAC, Mirza DF et al (2006) Hepatic artery thrombosis following orthotopic liver transplantation: a 10-year experience from a single centre in the United Kingdom. Liver Transpl 12(1):146–151
Warner P, Fusai G, Glantzounis GK, Sabin CA, Rolando N, Patch D et al (2011) Risk factors associated with early hepatic artery thrombosis after orthotopic liver transplantation–univariable and multivariable analysis. Transpl Int 24(4):401–408
Mergental H, Laing RW, Kirkham AJ, Perera MTPR, Boteon YL, Attard J et al (2020) Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat Commun 11(1):1–12
Pusztaszeri MP, Seelentag W, Bosman FT (2006) Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem 54(4):385–395
Neil DAH (1994) The effects of preservation and reperfusion injury on transplanted arteries: an ultrastructural and histological assessment. PhD Thesis, The University of Queensland
Davies PF (2009) Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Rev Cardiol 6(1):16
Cerra FB, Raza S, Andres GA, Siegel JH (1977) The endothelial damage of pulsatile renal preservation and its relationship to perfusion pressure and colloid osmotic pressure. Surgery 81(5):534–541
Lodge PA, Haisch CE, Huber SA, Martin B, Craighead JC (eds) (1991) Biological differences in endothelial cells depending upon organ derivation. Transplant Proc 23(1 Pt 1):216–218
Wagner WH, Henderson RM, Hicks HE, Banes AJ, Johnson G Jr (1988) Differences in morphology, growth rate, and protein synthesis between cultured arterial and venous endothelial cells. J Vasc Surg 8(4):509–519
Neil DAH, Lynch SV, Hardie IR, Effeney DJ (2002) Cold storage preservation and warm ischaemic injury to isolated arterial segments: endothelial cell injury. Am J Transplant 2(5):400–409
Morrison AD, BerwIck L, Orci L, Winegrad AI (1976) Morphology and metabolism of an aortic intima-media preparation in which an intact endothelium is preserved. J Clin Investig 57(3):650–660
Gottlob R (1977) The preservation of the venous endothelium by “dissection without touching” and by an atraumatic technique of vascular anastomosis. The importance for arterial and venous surgery. Minerva Chir 32(11):693–700
Haudenschild CC, Gould KE (1979) Vascular organ culture: prevention of endothelial damage due to removal and reperfusion. Scan Electron Microsc 3:865–872
Haudenschild C, Gould KE, Quist WC, LoGerfo FW (1981) Protection of endothelium in vessel segments excised for grafting. Circulation 64(2 Pt 2):II101–II107
Neil DAH, Lynch SV, Hardie IR, Effeney DJ (2000) Endothelium during microarterial graft procurement and transplantation. Microsurgery 20(3):121–125
Hangler HB, Pfaller K, Antretter H, Dapunt OE, Bonatti JO (2001) Coronary endothelial injury after local occlusion on the human beating heart. Ann Thorac Surg 71(1):122–127
McDonald AI, Iruela-Arispe ML (2015) Healing arterial ulcers: endothelial lining regeneration upon vascular denudation injury. Vascul Pharmacol 72:9–15
Rogers C, Parikh S, Seifert P, Edelman ER (1996) Endogenous cell seeding: remnant endothelium after stenting enhances vascular repair. Circulation 94(11):2909–2914
Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E et al (2002) Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Krüppel-like factor (KLF2). Blood 100(5):1689–1698
Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, Seppen J et al (2005) Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol 167(2):609–618
Atkins GB, Jain MK (2007) Role of Kruppel-like transcription factors in endothelial biology. Circ Res 100(12):1686–1695
Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN et al (2006) Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Investig 116(1):49–58
Parmar KM, Nambudiri V, Dai G, Larman HB, Gimbrone MA, García-Cardeña G (2005) Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J Biol Chem 280(29):26714–26719
Marrone G, Russo L, Rosado E, Hide D, García-Cardeña G, García-Pagán JC et al (2013) The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial–stellate cell deactivation induced by statins. J Hepatol 58(1):98–103
Eshmuminov D, Becker D, Bautista Borrego L, Hefti M, Schuler MJ, Hagedorn C et al (2020) An integrated perfusion machine preserves injured human livers for 1 week. Nat Biotechnol 38(2):189–198
Cheng A, Williamitis CA, Slaughter MS (2014) Comparison of continuous-flow and pulsatile-flow left ventricular assist devices: is there an advantage to pulsatility? Ann Cardiothorac Surg 3(6):573–581
Gambillara V, Thacher T, Silacci P, Stergiopulos N (2008) Effects of reduced cyclic stretch on vascular smooth muscle cell function of pig carotids perfused ex vivo. Am J Hypertens 21(4):425–431
Potapov EV, Dranishnikov N, Morawietz L, Stepanenko A, Rezai S, Blechschmidt C et al (2012) Arterial wall histology in chronic pulsatile-flow and continuous-flow device circulatory support. J Heart Lung Transplant 31(11):1171–1176
Cortese F, Ciccone MM, Gesualdo M, Iacoviello M, Frigerio M, Cipriani M et al (2021) Continuous flow left ventricular assist devices do not worsen endothelial function in subjects with chronic heart failure: a pilot study. ESC Heart Fail 8(5):3587–3593
Sneiders D, Houwen T, Pengel LH, Polak WG, Dor FJ, Hartog H (2018) Systematic review and meta-analysis of posttransplant hepatic artery and biliary complications in patients treated with transarterial chemoembolization before liver transplantation. Transplantation 102(1):88–96
Author information
Authors and Affiliations
Contributions
Conceptualization and design: JA, MTPRP, and DAHN; data collection, analysis, and data synthesis/interpretation: all authors; drafting of the manuscript: JA, DS, and DAHN; critically revising the manuscript: all authors.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Institutional Review Board of the Queen Elizabeth Hospital Birmingham (CARMS no. 15202).
Consent to participate
This study was observational and made use of residual donor materials and anonymised data; no specific additional consent was necessary for the current study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
J. Attard and D. Sneiders shared first authorship.
Rights and permissions
About this article
Cite this article
Attard, J., Sneiders, D., Laing, R. et al. The effect of end-ischaemic normothermic machine perfusion on donor hepatic artery endothelial integrity. Langenbecks Arch Surg 407, 717–726 (2022). https://doi.org/10.1007/s00423-021-02394-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-021-02394-4