Abstract
Purpose
This study aimed to assess the impact of neoadjuvant therapy (NAT) for borderline resectable or locally advanced pancreatic cancer (BR/LAPC) on the American Joint Commission on Cancer (AJCC) nodal status.
Methods
The medical records of BR/LAPC patients who underwent surgery with curative intent were retrospectively reviewed. The nodal status was compared between patients who underwent upfront surgery (UFS) and those who received NAT. Moreover, clinicopathological factors and prognostic factors for overall survival were analyzed.
Results
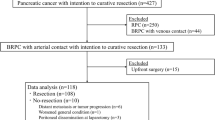
In all, 200 patients with BR/LAPC, 78 with UFS, and 122 with NAT were enrolled. The nodal status was significantly lower in patients after NAT than after UFS (p = 0.011). A multivariate analysis of overall survival showed that UFS (hazard ratio (HR) 1.61, p = 0.024) and N2 status (HR 2.69, p < 0.001) were independent poor prognostic factors. The median serum carbohydrate antigen (CA) 19–9 level after NAT in N2 patients was 105 U/mL, which was significantly higher than that of patients with N0 (p = 0.004) and N1 (p = 0.008) status.
Conclusion
Patients with BR/LAPC who underwent surgery after NAT had significantly lower N2 status and better prognosis than patients who underwent UFS. Elevated CA19-9 levels after NAT indicated a higher nodal status.




Similar content being viewed by others
Abbreviations
- CA 19-9:
-
Carbohydrate antigen 19–9
- AJCC:
-
American Joint Commission on Cancer
- NAT:
-
Neoadjuvant therapy
- UFS:
-
Upfront surgery
- BR/LAPC:
-
Borderline resectable or locally advanced pancreatic cancer
- FOLFIRINOX:
-
Fluorouracil/leucovorin/oxaliplatin/irinotecan
- MDCT:
-
Multi-detector row computed tomography
- GS:
-
Gemcitabine/S-1
- GAS:
-
Gemcitabine/nab-paclitaxel/S-1
- PV/SMV:
-
Portal or superior mesenteric vein
- OS:
-
Overall survival
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- IQR:
-
Interquartile range
- LNR:
-
Lymph node ratio
- MST:
-
Median survival time
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68(1):7–30
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74(11):2913–2921
Hackert T, Ulrich A, Buchler MW (2017) Can neoadjuvant therapy in pancreatic cancer increase the pool of patients eligible for pancreaticoduodenectomy? Adv Surg 51(1):1–10
Oba A, Croce C, Hosokawa P, Meguid C, Torphy RJ, Al-Musawi MH et al (2020) Prognosis based definition of resectability in pancreatic cancer: a road map to new guidelines. Ann Surg
Barnes CA, Chavez MI, Tsai S, Aldakkak M, George B, Ritch PS et al (2019) Survival of patients with borderline resectable pancreatic cancer who received neoadjuvant therapy and surgery. Surgery 166(3):277–285
Jang JY, Han Y, Lee H, Kim SW, Kwon W, Lee KH et al (2018) Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg 268(2):215–222
Asano D, Nara S, Kishi Y, Esaki M, Hiraoka N, Tanabe M et al (2019) A single-institution validation study of lymph node staging by the AJCC 8th edition for patients with pancreatic head cancer: a proposal to subdivide the N2 category. Ann Surg Oncol. 26(7):2112–20
Tol JA, Gouma DJ, Bassi C, Dervenis C, Montorsi M, Adham M et al (2014) Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 156(3):591–600
Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakashima A et al (2010) Number of metastatic lymph nodes, but not lymph node ratio, is an independent prognostic factor after resection of pancreatic carcinoma. J Am Coll Surg 211(2):196–204
Strobel O, Hinz U, Gluth A, Hank T, Hackert T, Bergmann F et al (2015) Pancreatic adenocarcinoma: number of positive nodes allows to distinguish several N categories. Ann Surg 261(5):961–969
Basturk O, Saka B, Balci S, Postlewait L, Knight J, Goodman M et al (2015) Substaging of lymph node status in resected pancreatic ductal adenocarcinoma has strong prognostic correlations: proposal for a revised n classification for TNM staging. Ann Surg Oncol 22(3):1187–1195
Kakar S, Pawlik TM, Allen PJ et al (2017) AJCC cancer staging manual, 8th edn. Springer-Verlag, New York, NY
van Roessel S, Kasumova GG, Verheij J, Najarian RM, Maggino L, de Pastena M et al (2018) International validation of the eighth edition of the American Joint Committee on Cancer (AJCC) TNM Staging System in Patients With Resected Pancreatic Cancer. JAMA Surg. 153(12):e183617
Kamarajah S, Burns WR, Frankel TL, Cho C, Nathan H (2017) Validation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with pancreatic adenocarcinoma: a Surveillance, Epidemiology and End Results (SEER) Analysis. Ann Surg Oncol 24(7):2023–30
Allen PJ, Kuk D, Castillo CF, Basturk O, Wolfgang CL, Cameron JL et al (2017) Multi-institutional validation study of the American Joint Commission on Cancer (8th Edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg. 265(1):185–91
Schlitter AM, Jesinghaus M, Jager C, Konukiewitz B, Muckenhuber A, Demir IE et al (2017) pT but not pN stage of the 8th TNM classification significantly improves prognostication in pancreatic ductal adenocarcinoma. Eur J Cancer 84:121–129
Michelakos T, Pergolini I, Castillo CF, Honselmann KC, Cai L, Deshpande V et al (2019) Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann Surg 269(4):733–740
Murakami Y, Uemura K, Sudo T, Hashimoto Y, Kondo N, Nakagawa N et al (2017) Survival impact of neoadjuvant gemcitabine plus S-1 chemotherapy for patients with borderline resectable pancreatic carcinoma with arterial contact. Cancer Chemother Pharmacol 79(1):37–47
Ielpo B, Caruso R, Duran H, Diaz E, Fabra I, Malave L et al (2017) A comparative study of neoadjuvant treatment with gemcitabine plus nab-paclitaxel versus surgery first for pancreatic adenocarcinoma. Surg Oncol 26(4):402–410
Fujii T, Yamada S, Murotani K, Kanda M, Sugimoto H, Nakao A et al (2015) Inverse probability of treatment weighting analysis of upfront surgery versus neoadjuvant chemoradiotherapy followed by surgery for pancreatic adenocarcinoma with arterial abutment. Medicine (Baltimore). 94(39):e1647
Tempero MA (2020) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Pancreatic Adenocarcinoma V.1.2020. National Comprehensive Cancer Network, Inc. Available at http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed 5 Nov 2020
Kondo N, Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakagawa N et al (2017) A phase 1 study of gemcitabine/nab-paclitaxel/S-1 (GAS) combination neoadjuvant chemotherapy for patients with locally advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol 79(4):775–781
Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364(19):1817–1825
Murakami Y, Uemura K, Hashimoto Y, Kondo N, Nakagawa N, Takahashi S et al (2016) Survival effects of adjuvant gemcitabine plus S-1 chemotherapy on pancreatic carcinoma stratified by preoperative resectability status. J Surg Oncol 113(4):405–412
Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Kondo N et al (2012) Long-term results of adjuvant gemcitabine plus S-1 chemotherapy after surgical resection for pancreatic carcinoma. J Surg Oncol 106(2):174–180
Truty MJ, Kendrick ML, Nagorney DM, Smoot RL, Cleary SP, Graham RP et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann Surg. https://doi.org/10.1097/SLA.0000000000003284
Perri G, Prakash L, Wang H, Bhosale P, Varadhachary GR, Wolff R et al. Radiographic and serologic predictors of pathologic major response to preoperative therapy for pancreatic cancer. Ann Surg. https://doi.org/10.1097/SLA.0000000000003442
Tzeng CW, Balachandran A, Ahmad M, Lee JE, Krishnan S, Wang H et al (2014) Serum carbohydrate antigen 19–9 represents a marker of response to neoadjuvant therapy in patients with borderline resectable pancreatic cancer. HPB (Oxford) 16(5):430–438
Combs SE, Habermehl D, Kessel KA, Bergmann F, Werner J, Naumann P et al (2014) Prognostic impact of CA 19–9 on outcome after neoadjuvant chemoradiation in patients with locally advanced pancreatic cancer. Ann Surg Oncol 21(8):2801–2807
Berger AC, Garcia M Jr, Hoffman JP, Regine WF, Abrams RA, Safran H et al (2008) Postresection CA 19–9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol 26(36):5918–5922
Tsai S, George B, Wittmann D, Ritch PS, Krepline AN, Aldakkak M et al (2020) Importance of normalization of CA19-9 levels following neoadjuvant therapy in patients with localized pancreatic cancer. Ann Surg 271(4):740–747
Murakami Y, Uemura K, Sudo T, Hashimoto Y, Kondo N, Nakagawa N et al (2017) Prognostic impact of normalization of serum tumor markers following neoadjuvant chemotherapy in patients with borderline resectable pancreatic carcinoma with arterial contact. Cancer Chemother Pharmacol 79(4):801–811
Williams JL, Kadera BE, Nguyen AH, Muthusamy VR, Wainberg ZA, Hines OJ et al (2016) CA19-9 Normalization during pre-operative treatment predicts longer survival for patients with locally progressed pancreatic cancer. J Gastrointest Surg 20:1331–1342
Aldakkak M, Christians KK, Krepline AN, George B, Ritch PS, Erickson BA et al (2015) Pre-treatment carbohydrate antigen 19–9 does not predict the response to neoadjuvant therapy in patients with localized pancreatic cancer. HPB (Oxford) 17(10):942–952
Kannagi R (2007) Carbohydrate antigen sialyl Lewis a – its pathophysiological significance and induction mechanism in cancer progression. Chang Gung Med J 30:189–209
Author information
Authors and Affiliations
Contributions
Study conception and design: Kenjiro Okada, Kenichiro Uemura, Naru Kondo, Yoshiaki Murakami, Shinya Takahashi. Acquisition of data: Kenjiro Okada, Tatsuaki Sumiyoshi, Shingo Seo, Hiroyuki Otsuka, Masahiro Serikawa, Yasutaka Ishii, Tomofumi Tsuboi. Analysis and interpretation of data: Kenjiro Okada, Kenichiro Uemura, Naru Kondo, Tatsuaki Sumiyoshi, Shingo Seo, Hiroyuki Otsuka, Masahiro Serikawa, Yasutaka Ishii, Tomofumi Tsuboi. Drafting of manuscript: Kenjiro Okada, Kenichiro Uemura, Naru Kondo. Critical revision of manuscript: Kenjiro Okada, Kenichiro Uemura, Naru Kondo, Yoshiaki Murakami, Shinya Takahashi.
Corresponding author
Ethics declarations
Definition of authorship
As per the guidelines of the International Committee of Medical Journal Editors (ICMJE), the authors met all of the following four criteria:
-
1.
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work.
-
2.
Drafting the work or revising it critically for important intellectual content.
-
3.
Final approval of the version to be published.
-
4.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Okada, K., Uemura, K., Kondo, N. et al. Neoadjuvant therapy contributes to nodal downstaging of pancreatic cancer. Langenbecks Arch Surg 407, 623–632 (2022). https://doi.org/10.1007/s00423-021-02339-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-021-02339-x