Abstract
Purpose
The aims of this study were to investigate the rate of intrathyroid extension of papillary thyroid microcarcinoma (PTMC) in patients operated for benign thyroid disease and to identify independent risk factors associated with it.
Methods
A retrospective study of 301 patients operated for benign thyroid diseases (hyperthyroid diseases, multinodular goitre, Hashimoto thyroiditis and benign thyroid tumours) was performed at a high-volume endocrine surgery unit of a tertiary referral academic hospital, in a 5-year period. These patients had a PTMC incidentally discovered on definite histopathological findings following total or near-total thyroidectomy. Since distinguishing between intrathyroid extension of PTMC as the result of intrathyroid dissemination or as the result of multicentricity is challenging, we observed them together as multifocality. In statistical analysis, we used standard descriptive statistics and univariate and multivariate logistic regression analysis to determine independent risk factors associated with multifocality.
Results
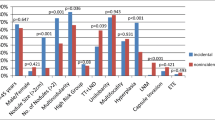
In our study, there were 85.4% females and 14.6% males with a median age of 54 years. A multinodular goitre (32.5%) was the most common indication for an operation. Most patients (68.4%) had a PTMC that was 5 mm or smaller. The most frequent histological variants of PTMC were the follicular variant (52.8%), followed by the papillary variant (22.6%) and the mixed follicular-papillary variant (18.6%). A multifocal PTMC was present in 26.6% of cases. An independent protective factor for multifocality of PTMC was a thyroid gland that weighed more than 38 g (OR 0.55, 95% CI 0.31–0.97, p = 0.039). Size of PTMC greater than 5 mm was an independent risk factor for a multifocal PTMC (OR 3.26, 95% CI 1.85–5.75, p = 0.000). Finally, the mixed follicular-papillary variant of PTMC represents an independent risk factor for a multifocal PTMC (OR 2.42, 95% CI 1.09–5.36, p = 0.030).
Conclusions
Intrathyroid extension is present in more than a quarter of PTMCs found in patients operated for benign thyroid disease. Independent risk factors for intrathyroid extension are size of PTMC greater than 5 mm and the mixed follicular-papillary variant of PTMC, while a large thyroid gland is an independent protective factor.


Similar content being viewed by others
References
El-Naggar AK, Chan JKC, Takata T, Grandis JR, Slootweg PJ (2017) The fourth edition of the head and neck World Health Organization blue book: editors’ perspectives. Hum Pathol 66:10–12
Slijepcevic N, Zivaljevic V, Paunovic I, Diklic A, Zivkovic P, Miljus D, Grgurevic A, Sipetic S (2016) Rising incidence of thyroid cancer in Serbia. Hippokratia 20(1):9–23
Bernet V (2010) Approach to the patient with incidental papillary microcarcinoma. J Clin Endocrinol Metab 95(8):3586–3592
Jung CK, Little MP, Lubin JH, Brenner AV, Wells SA Jr, Sigurdson AJ, Nikiforov YE (2014) The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab 99(2):E276–E285
Baloch ZW, Harrell RM, Brett EM, Randolph G, Garber JR, Committee AESS, Thyroid Scientific C (2017) American Association of Clinical Endocrinologists and American College of Endocrinology Disease state commentary: managing thyroid tumors diagnosed as noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Endocr Pract 23(9):1150–1155
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L (2016) 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid : Off J Am Thyroid Assoc 26(1):1–133
Roti E, degli Uberti EC, Bondanelli M, Braverman LE (2008) Thyroid papillary microcarcinoma: a descriptive and meta-analysis study. Eu J Endocrinol / Eur Fed Endocrine Soc 159(6):659–673
Slijepcevic N, Zivaljevic V, Marinkovic J, Sipetic S, Diklic A, Paunovic I (2015) Retrospective evaluation of the incidental finding of 403 papillary thyroid microcarcinomas in 2466 patients undergoing thyroid surgery for presumed benign thyroid disease. BMC Cancer 15(1):330
Tarasova VD, Tuttle RM (2017) Current management of low risk differentiated thyroid cancer and papillary microcarcinoma. Clin Oncol (R Coll Radiol) 29(5):290–297
Ito Y, Kudo T, Kihara M, Takamura Y, Kobayashi K, Miya A, Miyauchi A (2012) Prognosis of low-risk papillary thyroid carcinoma patients: its relationship with the size of primary tumors. Endocr J 59(2):119–125
Kaliszewski K, Strutynska-Karpinska M, Zubkiewicz-Kucharska A, Wojtczak B, Domoslawski P, Balcerzak W, Lukienczuk T, Forkasiewicz Z (2016) Should the prevalence of incidental thyroid cancer determine the extent of surgery in multinodular goiter? PLoS One 11(12):e0168654
Iida F, Yonekura M, Miyakawa M (1969) Study of intraglandular dissemination of thyroid cancer. Cancer 24(4):764–771
Melliere D, Hindie E, Becquemin JP, Desgranges P, Allaire E, Geachan E (2006) Differentiated thyroid carcinoma--how to improve the long-term results? Twenty-five-year outcomes of 850 patients. Bull Acad Natl Med 190(1):89–106 discussion 106-109
Can N, Tastekin E, Ozyilmaz F, Sezer YA, Guldiken S, Sut N, Sarikas N, Oz Puyan F, Guler B, Ayturk S, Celik M (2015) Histopathological evidence of lymph node metastasis in papillary thyroid carcinoma. Endocr Pathol 26(3):218–228
Hirokawa M, Kudo T, Ota H, Suzuki A, Miyauchi A (2016) Pathological characteristics of low-risk papillary thyroid microcarcinoma with progression during active surveillance. Endocr J 63(9):805–810
Hay ID, Grant CS, van Heerden JA, Goellner JR, Ebersold JR, Bergstralh EJ (1992) Papillary thyroid microcarcinoma: a study of 535 cases observed in a 50-year period. Surgery 112(6):1139–1146 discussion 1146-1137
Baudin E, Travagli JP, Ropers J, Mancusi F, Bruno-Bossio G, Caillou B, Cailleux AF, Lumbroso JD, Parmentier C, Schlumberger M (1998) Microcarcinoma of the thyroid gland: the Gustave-Roussy Institute experience. Cancer 83(3):553–559
Sugitani I, Fujimoto Y (1999) Symptomatic versus asymptomatic papillary thyroid microcarcinoma: a retrospective analysis of surgical outcome and prognostic factors. Endocr J 46(1):209–216
Noguchi S, Yamashita H, Murakami N, Nakayama I, Toda M, Kawamoto H (1996) Small carcinomas of the thyroid. A long-term follow-up of 867 patients. Arch Surg 131(2):187–191
Cady B, Rossi R (1988) An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery 104(6):947–953
Verburg FA, Mader U, Luster M, Reiners C (2009) Primary tumour diameter as a risk factor for advanced disease features of differentiated thyroid carcinoma. Clin Endocrinol 71(2):291–297
Pellegriti G, Scollo C, Lumera G, Regalbuto C, Vigneri R, Belfiore A (2004) Clinical behavior and outcome of papillary thyroid cancers smaller than 1.5 cm in diameter: study of 299 cases. J Clin Endocrinol Metab 89(8):3713–3720
Jukkola A, Bloigu R, Ebeling T, Salmela P, Blanco G (2004) Prognostic factors in differentiated thyroid carcinomas and their implications for current staging classifications. Endocr Relat Cancer 11(3):571–579
Baser H, Ozdemir D, Cuhaci N, Aydin C, Ersoy R, Kilicarslan A, Cakir B (2015) Hashimoto’s thyroiditis does not affect ultrasonographical, cytological, and histopathological features in patients with papillary thyroid carcinoma. Endocr Pathol 26:356–364
Lin JD (2010) Increased incidence of papillary thyroid microcarcinoma with decreased tumor size of thyroid cancer. Med Oncol 27(2):510–518
Kasai N, Sakamoto A (1987) New subgrouping of small thyroid carcinomas. Cancer 60(8):1767–1770
Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, Ito K, Takami H, Takanashi Y (2003) Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg 237(3):399–407
Chow SM, Law SC, Chan JK, Au SK, Yau S, Lau WH (2003) Papillary microcarcinoma of the thyroid-prognostic significance of lymph node metastasis and multifocality. Cancer 98(1):31–40
Roti E, Rossi R, Trasforini G, Bertelli F, Ambrosio MR, Busutti L, Pearce EN, Braverman LE, Degli Uberti EC (2006) Clinical and histological characteristics of papillary thyroid microcarcinoma: results of a retrospective study in 243 patients. J Clin Endocrinol Metab 91(6):2171–2178
Besic N, Pilko G, Petric R, Hocevar M, Zgajnar J (2008) Papillary thyroid microcarcinoma: prognostic factors and treatment. J Surg Oncol 97(3):221–225
Duran AO, Anil C, Gursoy A, Nar A, Altundag O, Inanc M, Bozkurt O, Tutuncu NB (2014) The relationship between thyroid volume and malignant thyroid disease. Med Oncol 31(1):814
Shi X, Liu R, Basolo F, Giannini R, Shen X, Teng D, Guan H, Shan Z, Teng W, Musholt TJ et al (2015) Differential clinicopathological risk and prognosis of major papillary thyroid cancer variants. J Clin Endocrinol Metab :jc20152917
Noguchi S, Yamashita H, Uchino S, Watanabe S (2008) Papillary microcarcinoma. World J Surg 32(5):747–753
Hay ID, Hutchinson ME, Gonzalez-Losada T, McIver B, Reinalda ME, Grant CS, Thompson GB, Sebo TJ, Goellner JR (2008) Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery 144(6):980–987 discussion 987-988
Mazzaferri EL (2007) Management of low-risk differentiated thyroid cancer. Endocr Pract 13(5):498–512
Roh JL, Kim JM, Park CI (2008) Central cervical nodal metastasis from papillary thyroid microcarcinoma: pattern and factors predictive of nodal metastasis. Ann Surg Oncol 15(9):2482–2486
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17(6):1471–1474
Cheema Y, Repplinger D, Elson D, Chen H (2006) Is tumor size the best predictor of outcome for papillary thyroid cancer? Ann Surg Oncol 13(11):1524–1528
Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI, Tuttle RM, American Thyroid Association Guidelines Taskforce (2006) Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid : Off J Am Thyroid Assoc 16(2):109–142
Wang TS, Goffredo P, Sosa JA, Roman SA: Papillary thyroid microcarcinoma: an over-treated malignancy? World journal of surgery 2014
American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated thyroid C, Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B et al (2009) Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid : Off J Am Thyroid Assoc 19(11):1167–1214
DeLellis R, Lloyd R, Heitz P, Eng C (2004) Pathology and genetics of tumors of endocrine organs. IARC Press, Lyon
Ito Y, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K, Yokozawa T, Matsuzuka F, Kuma S, Kuma K, Miyauchi A (2003) An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid : Off J Am Thyroid Assoc 13(4):381–387
Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, Tomoda C, Takamura Y, Kobayashi K, Miya A (2010) An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg 34(1):28–35
Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y (2010) Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg 34(6):1222–1231
Author information
Authors and Affiliations
Contributions
Study conception and design: NS, VZ; Acquisition of data: NS, MJ, BO; Analysis and interpretation of data: NS, VZ; Drafting of manuscript: NS, MJ, BO; Critical revision of manuscript: AD, IP.
Corresponding author
Ethics declarations
Conflict of interest
Author Nikola Slijepcevic declares that he has no conflict of interest. Author Vladan Zivaljevic declares that he has no conflict of interest. Author Aleksandar Diklic declares that he has no conflict of interest. Author Milan Jovanovic declares that he has no conflict of interest. Author Branislav Oluic declares that he has no conflict of interest. Author Ivan Paunovic declares that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Slijepcevic, N., Zivaljevic, V., Diklic, A. et al. Risk factors associated with intrathyroid extension of thyroid microcarcinomas. Langenbecks Arch Surg 403, 615–622 (2018). https://doi.org/10.1007/s00423-018-1680-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-018-1680-3