Abstract
Purpose
Altered expression and/or function of ribosomal RNA (rRNA)-binding proteins CUGBP2/CELF2 might influence post-transcriptional regulation of the HO-1- and COX-2-mediated cytoprotective pathways and represents an important therapeutic target. The aim of this study was to assess the effects of CUGBP2-mediated post-transcriptional regulation of COX-2 and HO-1 in pancreatic cancer cells in regard of response to gemcitabine (GEM) treatment.
Methods
Expression of CUGBP2, COX-2, and HO-1 was evaluated using qRT-PCR and Western blot methods. Cell viability after treatment with GEM and/or curcumin and siCUGBP2 was evaluated using MTT and crystal violet tests. RNA immunoprecipitation analysis was used to confirm COX-2 and HO-1 post-transcriptional regulation by CUGBP2 protein.
Results
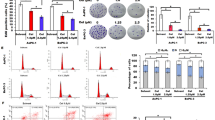
CUGBP2 expression at the messenger RNA (mRNA) level was 2.2-fold lower (p = 0.007), but HO-1 and COX-2 expression was increased 6.9- (p = 0.023) and 2.3- (p = 0.046) fold in pancreatic cancer tissues. The median survival of patients with low CUGBP2 expression from the lowest tercile was 13.8 months. The median survival of patients in terciles of middle and high CUGBP2 expression levels was 21.9 month (p = 0.123). Induction of CUGBP2 expression by curcumin resulted in the downregulation of HO-1 and COX-2 and strongly sensitized tumor cells to GEM treatment. However, CUGBP2 silencing upregulated HO-1 and COX-2 protein expression and had a high effect on cells viability.
Conclusion
Decreased activity of CUGBP2 could be associated with high chemoresistance and early dissemination of pancreatic cancer through the HO-1- and COX-2-mediated cytoprotective and carcinogenesis pathways. Curcumin significantly increased the effectiveness of GEM treatment in vitro via the CUGBP2-mediated post-transcriptional regulation pathway.








Similar content being viewed by others
Notes
References
Maris C, Dominguez C, Allain FH (2005) The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J 272(9):2118–2131. doi:10.1111/j.1742-4658.2005.04653.x
Sureban SM, Murmu N, Rodriguez P, May R, Maheshwari R, Dieckgraefe BK, Houchen CW, Anant S (2007) Functional antagonism between RNA binding proteins HuR and CUGBP2 determines the fate of COX-2 mRNA translation. Gastroenterology 132(3):1055–1065. doi:10.1053/j.gastro.2006.12.031
Ladd AN, Cooper TA (2004) Multiple domains control the subcellular localization and activity of ETR-3, a regulator of nuclear and cytoplasmic RNA processing events. J Cell Sci 117(Pt 16):3519–3529. doi:10.1242/jcs.01194
Singh G, Charlet BN, Han J, Cooper TA (2004) ETR-3 and CELF4 protein domains required for RNA binding and splicing activity in vivo. Nucleic Acids Res 32(3):1232–1241. doi:10.1093/nar/gkh275
Natarajan G, Ramalingam S, Ramachandran I, May R, Queimado L, Houchen CW, Anant S (2008) CUGBP2 downregulation by prostaglandin E2 protects colon cancer cells from radiation-induced mitotic catastrophe. Am J Physiol Gastrointest Liver Physiol 294(5):G1235–1244. doi:10.1152/ajpgi.00037.2008
Mukhopadhyay D, Houchen CW, Kennedy S, Dieckgraefe BK, Anant S (2003) Coupled mRNA stabilization and translational silencing of cyclooxygenase-2 by a novel RNA binding protein, CUGBP2. Mol Cell 11(1):113–126
Crane CH, Mason K, Janjan NA, Milas L (2003) Initial experience combining cyclooxygenase-2 inhibition with chemoradiation for locally advanced pancreatic cancer. Am J Clin Oncol 26(4):S81–84
Berberat PO, Dambrauskas Z, Gulbinas A, Giese T, Giese N, Kunzli B, Autschbach F, Meuer S, Buchler MW, Friess H (2005) Inhibition of heme oxygenase-1 increases responsiveness of pancreatic cancer cells to anticancer treatment. Clin Cancer Res 11(10):3790–3798. doi:10.1158/1078-0432.CCR-04-2159
Kanai M, Yoshimura K, Asada M, Imaizumi A, Suzuki C, Matsumoto S, Nishimura T, Mori Y, Masui T, Kawaguchi Y, Yanagihara K, Yazumi S, Chiba T, Guha S, Aggarwal BB (2011) A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol 68(1):157–164. doi:10.1007/s00280-010-1470-2
Kanai M (2014) Therapeutic applications of curcumin for patients with pancreatic cancer. World J Gastroenterol 20(28):9384–9391. doi:10.3748/wjg.v20.i28.9384
Swamy MV, Citineni B, Patlolla JM, Mohammed A, Zhang Y, Rao CV (2008) Prevention and treatment of pancreatic cancer by curcumin in combination with omega-3 fatty acids. Nutr Cancer 60(Suppl 1):81–89. doi:10.1080/01635580802416703
Half E, Arber N (2009) Colon cancer: preventive agents and the present status of chemoprevention. Expert Opin Pharmacother 10(2):211–219. doi:10.1517/14656560802560153
Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, Dou QP (2008) Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res 68(18):7283–7292. doi:10.1158/0008-5472.CAN-07-6246
Eom DW, Lee JH, Kim YJ, Hwang GS, Kim SN, Kwak JH, Cheon GJ, Kim KH, Jang HJ, Ham J, Kang KS, Yamabe N (2015) Synergistic effect of curcumin on epigallocatechin gallate-induced anticancer action in PC3 prostate cancer cells. BMB Rep 48(8):461–466
Terlikowska KM, Witkowska AM, Zujko ME, Dobrzycka B, Terlikowski SJ (2014) Potential application of curcumin and its analogues in the treatment strategy of patients with primary epithelial ovarian cancer. Int J Mol Sci 15(12):21703–21722. doi:10.3390/ijms151221703
Badrzadeh F, Akbarzadeh A, Zarghami N, Yamchi MR, Zeighamian V, Tabatabae FS, Taheri M, Kafil HS (2014) Comparison between effects of free curcumin and curcumin loaded NIPAAm-MAA nanoparticles on telomerase and PinX1 gene expression in lung cancer cells. Asian Pac J Cancer Prev 15(20):8931–8936
Vojdani A, Erde J (2006) Regulatory T cells, a potent immunoregulatory target for CAM researchers: modulating tumor immunity, autoimmunity and alloreactive immunity (III). Evid Based Complement Alternat Med 3(3):309–316. doi:10.1093/ecam/nel047
Abe Y, Hashimoto S, Horie T (1999) Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res 39(1):41–47. doi:10.1006/phrs.1998.0404
Mukhopadhyay D, Jung J, Murmu N, Houchen CW, Dieckgraefe BK, Anant S (2003) CUGBP2 plays a critical role in apoptosis of breast cancer cells in response to genotoxic injury. Ann N Y Acad Sci 1010:504–509
Hill R, Li Y, Tran LM, Dry S, Calvopina JH, Garcia A, Kim C, Wang Y, Donahue TR, Herschman HR, Wu H (2012) Cell intrinsic role of COX-2 in pancreatic cancer development. Mol Cancer Ther 11(10):2127–2137. doi:10.1158/1535-7163.MCT-12-0342
Dore M (2011) Cyclooxygenase-2 expression in animal cancers. Vet Pathol 48(1):254–265. doi:10.1177/0300985810379434
Shih RH, Yang CM (2010) Induction of heme oxygenase-1 attenuates lipopolysaccharide-induced cyclooxygenase-2 expression in mouse brain endothelial cells. J Neuroinflammation 7:86. doi:10.1186/1742-2094-7-86
Lin LC, Ho FM, Yen SJ, Wu PY, Hung LF, Huang WJ, Liang YC (2010) Carbon monoxide induces cyclooxygenase-2 expression through MAPKs and PKG in phagocytes. Int Immunopharmacol 10(12):1520–1525. doi:10.1016/j.intimp.2010.08.026
Gozzelino R, Jeney V, Soares MP (2010) Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 50:323–354. doi:10.1146/annurev.pharmtox.010909.105600
Abraham NG, Cao J, Sacerdoti D, Li X, Drummond G (2009) Heme oxygenase: the key to renal function regulation. Am J Physiol Renal Physiol 297(5):F1137–1152. doi:10.1152/ajprenal.90449.2008
Murmu N, Jung J, Mukhopadhyay D, Houchen CW, Riehl TE, Stenson WF, Morrison AR, Arumugam T, Dieckgraefe BK, Anant S (2004) Dynamic antagonism between RNA-binding protein CUGBP2 and cyclooxygenase-2-mediated prostaglandin E2 in radiation damage. Proc Natl Acad Sci U S A 101(38):13873–13878. doi:10.1073/pnas.0406066101
Otsuka N, Tsuritani K, Sakurai T, Kato K, Matoba R, Itoh J, Okuyama S, Yamada K, Yoneda Y (2009) Transcriptional induction and translational inhibition of Arc and Cugbp2 in mice hippocampus after transient global ischemia under normothermic condition. Brain Res 1287:136–145. doi:10.1016/j.brainres.2009.06.050
Ladd AN, Charlet N, Cooper TA (2001) The CELF family of RNA binding proteins is implicated in cell-specific and developmentally regulated alternative splicing. Mol Cell Biol 21(4):1285–1296. doi:10.1128/MCB.21.4.1285-1296.2001
Acknowledgments
We thank Dovile Gataveckaite and Augustina Gasianec (Vytautas Magnus University) for technical assistance and Vaidotas Cesna and Dr. Rima Ramonaite (Lithuanian University of Health Sciences) for the advice on experimental planning. We thank the EPZ-Pancobank team (supported by the Heidelberger Stiftung Chirurgie, BMBF grant 01GS08114 and BMBH-alliance/BMBF grant 01EY1101) for the provision of the expression and clinical data used for confirmation analyses.
Author contributions
Aldona Jakstaite carried out the molecular qRT-PCR studies and drafted the manuscript. Aurelija Maziukiene maintained cell cultures and conducted the transfection and curcumin stimulation experiments. Giedre Silkuniene performed the Western blot analysis. Kristina Kmieliute carried out the microscopic analysis. Albertas Dauksa and Saulius Paskauskas interpreted the data. Antanas Gulbinas conceived the study, participated in its design, and coordinated and helped to draft the manuscript. Zilvinas Dambrauskas designed and planned the study and performed the statistical data analysis. All authors have read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research is funded by the European Social Fund under the Global Grant measure (Project no. VP1-3.1-ŠMM-07-K-02-062).
Conflicts of interest
None.
Ethical approval
Ethical approval was issued by the Ethics Committee of the Lithuanian University of Health Sciences (No. BE-2-10 and BE-2-17). Consent for the use of routine blood samples, biopsies, and surgical tissue specimens for the research purposes was obtained from all the patients or their representatives. This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Jakstaite, A., Maziukiene, A., Silkuniene, G. et al. Upregulation of cugbp2 increases response of pancreatic cancer cells to chemotherapy. Langenbecks Arch Surg 401, 99–111 (2016). https://doi.org/10.1007/s00423-015-1364-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-015-1364-1