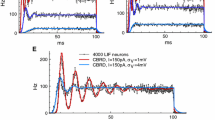
Abstract
The classical Hodgkin–Huxley (HH) point-neuron model of action potential generation is four-dimensional. It consists of four ordinary differential equations describing the dynamics of the membrane potential and three gating variables associated to a transient sodium and a delayed-rectifier potassium ionic currents. Conductance-based models of HH type are higher-dimensional extensions of the classical HH model. They include a number of supplementary state variables associated with other ionic current types, and are able to describe additional phenomena such as subthreshold oscillations, mixed-mode oscillations (subthreshold oscillations interspersed with spikes), clustering and bursting. In this manuscript we discuss biophysically plausible and phenomenological reduced models that preserve the biophysical and/or dynamic description of models of HH type and the ability to produce complex phenomena, but the number of effective dimensions (state variables) is lower. We describe several representative models. We also describe systematic and heuristic methods of deriving reduced models from models of HH type.








Similar content being viewed by others
References
Abbott LF (1999) Lapicque’s introduction of the integrate-and-fire model neuron (1907). Brain Res Bull 50:303–304
Acker CD, Kopell N, White JA (2003) Synchronization of strongly coupled excitatory neurons: Relating network behavior to biophysics. J Comput Neurosci 15:71–90
Akman O, Schaefer E (2015) An evolutionary computing approach for parameter estimation investigation of a model for cholera. J Biol Dyn 9:147–158
Baer SM, Erneux T, Rinzel J (1989) The slow passage through a Hopf bifurcation: delay, memory effects, and resonance. SIAM J Appl Math 49:55–71
Barranca VJ, Johnson DC, Moyher JL, Sauppe JP, Shkarayev MS, Kovacic G, Cai D (2013) Dynamics of the exponential integrate-and-fire model with slow currents and adaptation. J Comput Neurosci 37:161–180
Bertram R, Butte MJ, Kiemel T, Sherman A (1995) Topological and phenomenological classification of bursting oscillations. Bull Math Biol 57:413–439
Bonhoeffer K (1948) Activation of passive iron as a model for the excitation of nerve. J Gen Physiol 32:69–91
Borgers C (2017) An introduction to modeling neuronal dynamics. Springer
Brette R, Gerstner W (2005) Adaptive exponential integrate-and-fire model as an effective description of neuronal activity. J Neurophysiol 94:3637–3642
Brøns M, Kaper TJ, Rotstein HG (2008) Introduction to focus issue: mixed mode oscillations: experiment, computation, and analysis. Chaos 18:015101
Brunel N, van Rossum MCW (2007) Lapicque’s 1907 paper: from frogs to integrate-and-fire. Biol Cybern 97:337–339
Brunton SL, Proctor JL, Kutz JN (2016) Discovering governing equations from data by sparse identification of nonlinear dynamical systems. Proc Natl Acad Sci USA 113:3932–3937
Burkitt AN (2006) A review of the integrate-and-fire neuron model: I. homogeneous synaptic input. Biol Cybern 95:1–19
Butera RJ, Rinzel J, Smith JC (1999) Models of respiratory rhythm generation in the pre-Botzinger complex. I. bursting pacemaker neurons. J Neurophysiol 82:382–397
Chamption K, Lusch B, Kutz NJ, Brunton SL (2019) Data-driven discovery of coordinates and governing equations. Proc Natl Acad Sci USA 116:22445–22451
Coombes S, Bressloff PC (1999) Mode locking and arnold tongues in integrate-and-fire neural oscillators. Phys Rev E 60:2086–2096
Dayan P, Abbott LF (2001) Theoretical neuroscience. The MIT Press, Cambridge
Deb K (2001) Multi-objective optimization using evolutionary algorithms. Wiley, Hoboken
Deb K, Beyer H-G (2001) Self-adaptive genetic algorithms with simulated binary crossover. Evol Comput 9:197–221
Deb K, Anand A, Joshe D (2002) A computationally efficient evolutionary algorithm for real-parameter optimization. Evol Comput 10:371–395
Deb K, Pratap A, Agarwal S, Meyarivan T (2002) A fast and elitist multiobjective genetic algorithm: Nsga-ii. IEEE Trans Evolut Comput 6:182–197
Edelman GM, Gally JA (2001) Degeneracy and complexity in biological systems. Proc Natl Acad Sci USA 98:13763–13768
Ermentrout GB (1996) Type I membranes, phase resetting curves, and synchrony. Neural Comput 8:979–1001
Ermentrout GB, Kopell N (1986) Parabolic bursting in an excitable system coupled with a slow oscillation. SIAM J Appl Math 46:233–253
Ermentrout BG, Kopell N (1998) Fine structure of neural spiking and synchronization in the presence of conduction delays. Proc Natl Acad Sci USA 95:1259–1264
Ermentrout GB, Terman D (2010) Mathematical foundations of neuroscience. Springer
Evensen G (2009) Data assimilation: the ensemble Kalman filter. Springer
FitzHugh R (1960) Thresholds and plateaus in the Hodgkin-Huxley nerve equations. J Gen Physiol 43:867–896
FitzHugh R (1961) Impulses and physiological states in models of nerve membrane. Biophysical J 1:445–466
Fourcaud-Trocme N, Hansel D, van Vreeswijk C, Brunel N (2003) How spike generation mechanisms determine the neuronal response to fluctuating input. J Neurosci 23:11628–11640
Fransén E, Alonso AA, Dickson CT, Magistretti ME, Hasselmo J (2004) Ionic mechanisms in the generation of subthreshold oscillations and action potential clustering in entorhinal layer II stellate neurons. Hippocampus 14:368–384
Fuortes MGF, Mantegazzini F (1962) Interpretation of the repetitive firing of nerve cells. J Gen Physiol 45:1163–1179
Gabbiani F, Cox SJ (2017) Mathematics for neuroscientists, 2nd edn. Academic Press
Gerstner W, Kistler WM (2002) Spiking neuron models. Cambridge University Press
Gerstner W, Kistler WM, Naud R, Paninski L (2014) Neuronal dynamics: from single neurons to networks and models of cognition. Cambridge University Press
Goaillard JM, Marder E (2021) Ion channel degeneracy, variability, and covariation in neuron and circuit resilience. Annu Rev Neurosci 44:335–357
Golomb D (2014) Mechanism and function of mixed-mode oscillations in vibrissa motoneurons. PLoS ONE 9:e109205
Golomb D, Yue C, Yaari Y (2006) Contribution of persistent Na\(^+\) current and M-Type K\(^+\) current to somatic bursting in ca1 pyramidal cells: combined experimental and modeling study. J Neurophysiol 96:1912–1926
Goncalves PJ, Lueckmann J-M, Deistler M, Nonnenmacher M, Ocal K, Bassetto G, Chintaluri C, Podlaski WF, Haddad SA, Vogels TP, Greenberg DS, Macke JH (2020) Training deep neural density estimators to identify mechanistic models of neural dynamics. Elife 9:e56261
Hansel D, Mato G (2001) Existence and stability of persistent states in large neuronal networks. Phys Rev Lett 86:4175–4178
Hansel D, Mato G, Meunier C (1995) Synchrony in excitatory neural networks. Neural Comput 7:307–337
Hill AV (1936) Excitation and accommodation in nerve. Proc R Soc B 119:305–255
Hindmarsh JL, Rose RM (1994) A model for rebound bursting in mammalian neurons. Philos Trans R Soc Lond B 346:129–150
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conductance and excitation in nerve. J Physiol 117:500–544
Hodgkin AL, Huxley AF (1952) Currents carried by sodium and potassium ions through the membrane of the giant axon of loligo. J Physiol 116:449–472
Hutcheon B, Yarom Y (2000) Resonance, oscillations and the intrinsic frequency preferences in neurons. Trends Neurosci 23:216–222
Izhikevich EM (2001) Resonate-and-fire neurons. Neural Netw 14:883–894
Izhikevich EM (2003) Simple model of spiking neurons. IEEE Trans Neural Netw 14:1569–1572
Izhikevich E (2006) Dynamical Systems in Neuroscience: the geometry of excitability and bursting. MIT Press, Cambridge
Izhikevich EM (2010) Hybrid spiking models. Philos Trans R Soc A 368:5061–5070
Jalics J, Krupa M, Rotstein HG (2010) A novel mechanism for mixed-mode oscillations in a neuronal model. Dyn Syst Int J 25:445–482
Johnston D, Wu SM-S (1995) Foundations of cellular neurophysiology. The MIT Press, Cambridge
Jolivet R, Lewis TJ, Gerstner W (2004) Generalized integrate-and-fire models of neuronal activity approximate spike trains of a detailed model to a high degree of accuracy. J Neurophysiol 92:959–976
Kass RE, Amari S-I, Arai K, Brown EN, Diekman CO, Diesmann M, Doiron B, Eden UT, Fairhall A, Fiddyment GM, Fukai T, Grün S, Harrison M, Helias M, Kramer MA, Nakahara H, Teramae J-N, Thomas PJ, Reimers M, Rodu J, Rotstein HG, Shea-Brown E, Shimazaki H, Shinomoto S, Yu BM (2018) Computational neuroscience: mathematical and statistical perspectives. Annu Rev Stat 5:183–214
Kepler TB, Marder E, Abbott LF (1990) The effect of electrical coupling on the frequency of model neuronal oscillators. Science 248:83–85
Kistler WM, Gerstner W, van Hemmen JL (1997) Reduction of the Hodgkin-Huxley equations to a single-variable threshold model. Neural Comput 9:1015–1045
Knight BW (1972) Dynamics of encoding in a population of neurons. J Gen Physiol 59:734–766
Koch C (1999) Biophysics of computation. Oxford University Press
Krupa M, Szmolyan P (2001) Relaxation oscillation and canard explosion. J Differ Equ 174:312–368
Lapicque L (1907) Recherches quantitatives sur l’excitation électrique des nerfes traitée comme une polarization. J Physiol Pathol Gen 9:620–637
Latham PE, Richmond BJ, Nelson PG, Nirenberg S (2000) Intrinsic dynamics in neuronal networks. I. Theory. J Neurophysiol 83:808–827
Lecar H (2007) Morris-lecar model. Scholarpedia 2:1333
Lederman D, Patel R, Itani O, Rotstein HG (2022) Parameter estimation in the age of degeneracy and unidentifiability. Mathematics 10:170
Levenstein D, Alvarez VA, Amarasingham A, Azab H, Gerkin RC, Hasenstaub A, Iyer R, Jolivet R, Marzen S, Monaco JD, Prinz A, Quarishi S, Santamaría F, Shivkumar S, Singh MF, Stockton DB, Traub R, Rotstein HG, Nadim F, Redish D (2020) On the role of theory and modeling in neuroscience. arXiv preprint arXiv:2003.13825
Lillacci G, Khammash M (2010) Parameter estimation and model selection in computational biology. PLoS Comput Biol 6:e1000696
Manor Y, Rinzel J, Segev I, Yarom Y (1997) Low-amplitude oscillations in the inferior olive: A model based on electrical coupling of neurons with heterogeneous channel densities. J Neurophysiol 77:2736–2752
Marder E (2011) Variability, compensation, and modulation in neurons and circuits. Proc Natl Acad Sci USA 108:15542–15548
McCormick DA, Shu Y, Yu Y (2007) Hodgkin and Huxley model - still standing? Nature 445:E1–E2
Meng XY, Huguet G, Rinzel J (2012) Type III excitability, slope sensitivity and coincidence detection. Discrete Continuous Dyn Syst A 32:2729–2757
Mensi S, Naud R, Pozzorini C, Avermann M, Petersen CC, Gerstner J (2012) Parameter extraction and classification of three cortical neuron types reveals two distinct adaptation mechanisms. J Neurophysiol 107:1756–1775
Miller P (2018) An introductory course in computational neuroscience. MIT Press, Cambridge
Morris H, Lecar C (1981) Voltage oscillations in the barnacle giant muscle fiber. Biophysical J 35:193–213
Moye MJ, Diekman C (2018) Data assimilation methods for neuronal state and parameter estimation. J Math Neurosci 8:11
Nagumo JS, Arimoto S, Yoshizawa S (1962) An active pulse transmission line simulating nerve axon. Proc IRE 50:2061–2070
Naundorf B, Wolf F, Volgushev M (2006) Unique features of action potential initiation in cortical neurons. Nature 440:1060–1063
Papamarkou T, Hinkle J, Young JT, Womble D (2019) Challenges in Bayesian inference via markov chain monte carlo for neural networks. arXiv
Pena RFO, Rotstein HG (2022) Oscillations and variability in neuronal systems: interplay of autonomous transient dynamics and fast deterministic fluctuations. J Comput Neurosci
Perkel DH, Mulloney B, Budelli RW (1981) Quantitative methods for predicting neuronal behavior. Neuroscience 6:823–837
Pozzorini C, Mensi S, Hagens O, Naud R, Koch C, Gerstner W (2015) Automated high-throughput characterization of single neurons by means of simplified spiking models. PLoS Comput Biol 11:e1004275
Prescott SA, De Koninck Y, Sejnowski TJ (2008) Biophysical basis for three distinct dynamical mechanisms of action potential initiation. PLoS Comp. Biol. 4:e100198
Prescott SA, Ratté S, De Koninck Y, Sejnowski TJ (2008) Pyramidal neurons switch from integrator in vitro to resonators under in vivo-like conditions. J Neurophysiol 100:3030–3042
Prinz AA, Bucher D, Marder E (2004) Similar network activity from disparate circuit parameters. Nature Neurosci. 7:1345–1352
Prinz AA, Abbott LF, Marder E (2004) The dynamic clamp comes of age. Trends Neurosci 27:218–224
Richardson MJE, Brunel N, Hakim V (2003) From subthreshold to firing-rate resonance. J Neurophysiol 89:2538–2554
Rinzel J (1986) A formal classification of bursting mechanisms in excitable systems. In: Proceedeings of the international congress of mathematicians, pp 1578–1593
Rinzel J (1985) Bursting oscillations in an excitable membrane model. In: Sleeman BD, Jarvis RJ (eds) Ordinary and partial differential equations lecture notes in mathematics, vol 1151. Springer, Berlin, pp 304–316
Rinzel J (1985) Excitation dynamics: Insights from simplified membrane models. Fed Proc 44:2944–2946
Rinzel J, Ermentrout GB (1998) Analysis of neural excitability and oscillations. In: Koch C, Segev I (eds) Methods in neural modeling, 2nd edn. MIT Press, Cambridge, pp 251–292
Rossi RJ (2018) Mathematical statistics: an introduction to likelihood based inference. Wiley, New York
Rotstein HG, Nadim F (2020) Neurons and neural networks: computational models. In: Encyclopedia of life sciences. Wiley, Chichester
Rotstein HG (2013) Abrupt and gradual transitions between low and hyperexcited firing frequencies in neuronal models with fast synaptic excitation: A comparative study. Chaos 23:046104
Rotstein HG (2014) Frequency preference response to oscillatory inputs in two-dimensional neural models: a geometric approach to subthreshold amplitude and phase resonance. J Math Neurosci 4:11
Rotstein HG (2015) Subthreshold amplitude and phase resonance in models of quadratic type: nonlinear effects generated by the interplay of resonant and amplifying currents. J Comput Neurosci 38:325–354
Rotstein HG (2017) Spiking resonances in models with the same slow resonant and fast amplifying currents but different subthreshold dynamic properties. J Comput Neurosci 43:243–271
Rotstein HG (2017) Resonance modulation, annihilation and generation of antiresonance and antiphasonance in 3d neuronal systems: interplay of resonant and amplifying currents with slow dynamics. J Comput Neurosci 43:35–63
Rotstein HG (2017) The shaping of intrinsic membrane potential oscillations: positive/negative feedback, ionic resonance/amplification, nonlinearities and time scales. J Comput Neurosci 42:133–166
Rotstein HG (2018) Subthreshold resonance and phasonance in single cells: 2D models. In: Jaeger D, Jung R (eds) Encyclopedia of computational neuroscience: Springer Reference (www.springerreference.com). Springer, New York
Rotstein HG, Nadim F (2014) Frequency preference in two-dimensional neural models: a linear analysis of the interaction between resonant and amplifying currents. J Comput Neurosci 37:9–28
Rotstein HG, Oppermann T, White JA, Kopell N (2006) The dynamic structure underlying subthreshold oscillatory activity and the onset of spikes in a model of medial entorhinal cortex stellate cells. J Comput Neurosci 21:271–292
Rotstein HG, Wechselberger M, Kopell N (2008) Canard induced mixed-mode oscillations in a medial entorhinal cortex layer II stellate cell model. SIAM J Appl Dyn Syst 7:1582–1611
Rotstein HG, Coombes S, Gheorghe AM (2012) Canard-like explosion of limit cycles in two-dimensional piecewise-linear models of FitzHugh-Nagumo type. SIAM J Appl Dyn Syst 11:135–180
Senov A, Granichin O (2017) Projective approximation based gradient descent modification. IFAC-PapersOnLine 50:3899–3904
Shahriari B, Swersky K, Wang Z, Adams RP, de Freitas N (2016) Taking the human out of the loop: a review of Bayesian optimization. Proc IEEE 104:148–175
Sharp AA, O’Neil MB, Abbott LF, Marder E (1993) The dynamic clamp: artificial conductances in biological neurons. Trends Neurosci 16:389–394
Sharp AA, O’Neil MB, Abbott LF, Marder E (1993) Dynamic clamp: computer-generated conductances in real neurons. J Neurophysiol 69:992–995
Smith GD, Cox CL, Sherman SM, Rinzel J (2000) Fourier analysis of sinusoidally driven thalamocortical relay neurons and a minimal integrate-and-fire-or-burst model. J Neurophysiol 83:588–610
Stein RB (1965) A theoretical analysis of neuronal variability. Biophysical J 5:173–194
Stein RB (1967) Some models of neuronal variability. Biophysical J 7:37–68
Strogatz SH (1994) Nonlinear dynamics and Chaos. Addison Wesley, Reading MA
Szmolyan P, Wechselberger M (2001) Canards in r\(^{3} \). J Differ Equ 177:419–453
Taylor AL, Marder E (2011) Multiple models to capture the variability in biological neurons and networks. Nat Neurosci 14:133–138
Teeter C, Iyer R, Menon V, Gouwens N, Feng D, Berg J, Szafer Z, Cain H, Zeng N, Hawrylycz M, Koch C, Mihalas S (2018) Generalized leaky integrate-and-fire models classify multiple neuron types. Nat Commun 9:709
Torben-Nielsen B, Segev I, Yarom Y (2012) The generation of phase differences and frequency changes in a network model of inferior olive subthreshold oscillations. PLoS Comput Biol 8:31002580
Touboul J (2008) Bifurcation analysis of a general class of nonlinear integrate-and-fire neurons. SIAM J Appl Math 68:1045-1079
Treves A (1993) Mean-field analysis of neuronal spike dynamics. Network 4:259–284
Tuckwell HC (1988) Introduction to theoretical neurobiology, vol 2. Cambridge University Press
Turnquist AGR, Rotstein HG (2018) Quadratization: from conductance-based models to caricature models with parabolic nonlinearities. In: Jaeger D, Jung R (eds) Encyclopedia of computational neuroscience. Springer-Verlag, New York
van der Pol B (1920) A theory of the amplitude of free and forced triode oscillations. Radio Rev 1(701–710):754–762
van Geit W, De Schutter E, Achard P (2008) Automated neuron model optimization techniques: a review. Biol Cybern 99:241–251
Walter E, Pronzato L (1997) Identification of parametric models from experimental data. Springer, London
Wang X-J, Buzsáki G (1996) Gamma oscillations by synaptic inhibition in an interneuronal network model. J Neurosci 16:6402–6413
Wang X-J, Rinzel J (1992) Alternating and synchronous rhythms in reciprocally inhibitory model neurons. Neural Comput 4:84–97
Wechselberger M (2005) Existence and bifurcation of canards in R\(^{3} \) in the case of a folded node. SIAM J Appl Dyn Syst 4:101–139
Young G (1937) Note on excitation theories. Psychometrika 2:103–106
Acknowledgements
The authors acknowledge support from the NSF Grants CRCNS-DMS-1608077 (HGR) and IOS-2002863 (HGR), from CONICET, Argentina (UC), and the Fulbright Program (VGB). The authors are thankful to John Rinzel for useful suggestions and discussions. This paper benefited from discussions held as part of the workshop “Current and Future Theoretical Frameworks in Neuroscience” (San Antonio, TX, Feb 4–8, 2019) supported by the NSF Grants DBI-1820631 (HGR) and IOS-1516648 (Fidel Santamaría, co-organizer). This paper also benefited from discussions during the course on “Reduced and simplified spiking neuron models” taught at the VIII Latin American School on Computational Neuroscience (LASCON 2020) organized by Antonio Roque (USP, Brazil) and supported by FAPESP Grants 2013/07699-0 (NeuroMat) and 2019/10496-0 and the IBRO-LARC Schools Funding Program. The authors are grateful to an anonymous reviewer for useful comments and suggestions.
Author information
Authors and Affiliations
Contributions
HGR conceived the research and the manuscript. All authors wrote the main manuscript, prepared figures and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Benjamin Lindner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chialva, U., González Boscá, V. & Rotstein, H.G. Low-dimensional models of single neurons: a review. Biol Cybern 117, 163–183 (2023). https://doi.org/10.1007/s00422-023-00960-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-023-00960-1