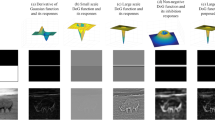
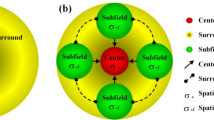
Abstract
Simple cells in primary visual cortex are believed to extract local contour information from a visual scene. The 2D Gabor function (GF) model has gained particular popularity as a computational model of a simple cell. However, it short-cuts the LGN, it cannot reproduce a number of properties of real simple cells, and its effectiveness in contour detection tasks has never been compared with the effectiveness of alternative models. We propose a computational model that uses as afferent inputs the responses of model LGN cells with center–surround receptive fields (RFs) and we refer to it as a Combination of Receptive Fields (CORF) model. We use shifted gratings as test stimuli and simulated reverse correlation to explore the nature of the proposed model. We study its behavior regarding the effect of contrast on its response and orientation bandwidth as well as the effect of an orthogonal mask on the response to an optimally oriented stimulus. We also evaluate and compare the performances of the CORF and GF models regarding contour detection, using two public data sets of images of natural scenes with associated contour ground truths. The RF map of the proposed CORF model, determined with simulated reverse correlation, can be divided in elongated excitatory and inhibitory regions typical of simple cells. The modulated response to shifted gratings that this model shows is also characteristic of a simple cell. Furthermore, the CORF model exhibits cross orientation suppression, contrast invariant orientation tuning and response saturation. These properties are observed in real simple cells, but are not possessed by the GF model. The proposed CORF model outperforms the GF model in contour detection with high statistical confidence (RuG data set: p < 10−4, and Berkeley data set: p < 10−4). The proposed CORF model is more realistic than the GF model and is more effective in contour detection, which is assumed to be the primary biological role of simple cells.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Albrecht DG, De Valois RL, Thorell LG (1980) Visual cortical-neurons—are bars or gratings the optimal stimuli. Science 207(4426): 88–90
Andrews BW, Pollen DA (1979) Relationship between spatial-frequency selectivity and receptive-field profile of simple cells. J Physiol 287: 163–176
Azzopardi G, Petkov N (2012) Trainable COSFIRE filters for keypoint detection and pattern recognition. IEEE Trans Pattern Anal Mach Intell (to appear)
Canny J (1986) A computational approach to edge-detection. IEEE Trans Pattern Anal Mach Intell 8(6): 679–698
Chung S, Ferster D (1998) Strength and orientation tuning of the thalamic input to simple cells revealed by electrically evoked cortical suppression. Neuron 20(6): 1177–1189
Croner LJ, Kaplan E (1995) Receptive-fields of P-ganglion and M-ganglion cells across the primate retina. Vis Res 35(1): 7–24
Daugman JG (1985) Uncertainty relation for resolution in space, spatial-frequency, and orientation optimized by two-dimensional visual cortical filters. J Opt Soc Am A 2(7): 1160–1169
de Boer R, Kuyper P (1968) Triggered correlation. IEEE Trans Bio-med Eng 15(3): 169–179
De Valois RL, Albrecht DG, Thorell LG (1978) Cortical cells: bar and edge detectors, or spatial frequency filters? In: Cool SJ, Smith I E L (eds) Frontiers in visual science, Springer-Verlag, Berlin, West Germany pp 544–556
De Valois KK, De Valois RL, Yund EW (1979) Responses of striate cortex cells to grating and checkerboard patterns. J Physiol 291: 483–505
De Valois RL, Yund EW, Hepler N (1982) The orientation and direction selectivity of cells in macaque visual-cortex. Vis Res 22(5): 531–544
DeAngelis GC, Ohzawa I, Freeman RD (1995) Receptive-field dynamics in the central visual pathways. Trends Neurosci 18(10): 451–458
DuBuf JMH (1993) Responses of simple cells—events, interferences, and ambiguities. Biol Cybernet 68(4): 321–333
Ferster D, Chung S, Wheat H (1996) Orientation selectivity of thalamic input to simple cells of cat visual cortex. Nature 380(6571): 249–252
Finn IM, Priebe NJ, Ferster D (2007) The emergence of contrast-invariant orientation tuning in simple cells of cat visual cortex. Neuron 54(1): 137–152
Gabor D (1946) Theory of communication. J Inst Electr Eng 93: 429–457
Glezer VD, Tsherbach TA, Gauselman VE, Bondarko VM (1980) Linear and non-linear properties of simple and complex receptive-fields in area-17 of the cat visual-cortex—a model of the field. Biological Cybernetics 37(4): 195–208
Grigorescu C, Petkov N, Westenberg MA (2004) Contour and boundary detection improved by surround suppression of texture edges. Image Vis Comput 22(8): 609–622
Grigorescu C, Petkov N, Westenberg MA (2003) Contour detection based on nonclassical receptive field inhibition. IEEE Trans Image Process 12(7): 729–739
Heitger F (1995) Feature detection using suppression and enhancement. Communication Technology Laboratory, Swiss Federal Institute of Technology, Lausanne, Technical Report TR-163
Hubel DH (1982) Exploration of the primary visual-cortex, 1955–78. Nature 299(5883): 515–524
Hubel DH, Wiesel TN (1962) Receptive fields, binocular interaction and functional architecture in cats visual cortex. J Physiol 160(1):106–154
Hubel DH, Wiesel TN (1974) Sequence regularity and geometry of orientation columns in monkey striate cortex. J Comp Neurol 158(3): 267–294
Irvin GE, Casagrande VA, Norton TT (1993) Center surround relationships of magnocellular, parvocellular, and koniocellular relay cells in primate lateral geniculate nucleus. Vis Neurosci 10(2): 363–373
Jones JP, Palmer LA (1987) An evaluation of the two-dimensional gabor filter model of simple receptive-fields in cat striate cortex. J Neurophysiol 58(6): 1233–1258
Kovesi P (1999) Image features from phase congruency. Videre 1(3)
Kulikowski JJ, Bishop PO (1981) Fourier-analysis and spatial representation in the visual-cortex. Experientia 37(2): 160–163
Macleod IDG, Rosenfeld A (1974) Visibility of gratings—spatial frequency channels or bar-detecting units. Vis Res 14(10): 909–915
Maffei L, Morrone C, Pirchio M, Sandini G (1979) Responses of visual cortical-cells to periodic and non-periodic stimuli. J Physiol 296: 27–47
Marcelja S (1980) Mathematical-description of the responses of simple cortical-cells. J Opt Soc Am 70(11): 1297–1300
Martin D, Fowlkes C, Tal D, Malik J (2001) A database of human segmented natural images and its application to evaluating segmentation algorithms and measuring ecological statistics. In: Proceedings of the 8th international conference computer vision, vol 2, pp 416–423
Martin DR, Fowlkes CC, Malik J (2004) Learning to detect natural image boundaries using local brightness, color, and texture cues. IEEE Trans Pattern Anal Mach Intell 26(5): 530–549
Mehrotra R, Namuduri KR, Ranganathan N (1992) Gabor filter-based edge-detection. Pattern Recogn 25(12): 1479–1494
Morrone MC, Burr DC (1988) Feature detection in human-vision—a phase-dependent energy-model. Proc R Soc Lond B 235(1280): 221–245
Morrone MC, Owens RA (1987) Feature detection from local energy. Pattern Recogn Lett 6(5): 303–313
Movshon JA, Thompson ID, Tolhurst DJ (1978a) Receptive-field organization of complex cells in cats striate cortex. J Physiol 283: 79–99
Petkov N (1995) Biologically motivated computationally intensive approaches to image pattern-recognition. Future Gener Comput Syst 11(4–5): 451–465
Priebe NJ, Ferster D (2006) Mechanisms underlying cross-orientation suppression in cat visual cortex. Nat Neurosci 9(4): 552–561
Reid RC, Alonso JM (1995) Specificity of monosynaptic connections from thalamus to visual-cortex. Nature 378(6554): 281–284
Ringach D, Shapley R (2004) Reverse correlation in neurophysiology. Cogn Sci 28(2): 147–166
Rodieck RW (1965) Quantitative analysis of cat retinal ganglion cell response to visual stimuli. Vision Res 5(11): 583–601
Rosenthaler L, Heitger F, Kubler O, von der Heydt R (1992) Detection of general edges and keypoints. In: Sandini G (ed) Proceedings of the European Conference Computer Vision (ECCV92), pp 78–86
Sclar G, Freeman R (1982) Orientation selectivity in the cats striate cortex is invariant with stimulus contrast. Exp Brain Res 46(3): 457–461
Sclar G, Maunsell JHR, Lennie P (1990) Coding of image-contrast in central visual pathways of the macaque moneky. Vision Res 30(1): 1–10
Shin MC, Goldgof D, Bowyer KW (1998) An objective comparison methodology of edge detection algorithms using a structure from motion task. In: Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition, 1998 (Cat. No.98CB36231), pp 190–195
Sonka M, Hlavac V, Boyle R (1999) Image processing, analysis, and machine vision. Brooks/Cole, Pacific Grove
Stork DG, Wilson HR (1990) Do gabor functions provide appropriate descriptions of visual cortical receptive-fields. J Opt Soc Am A 7(8): 1362–1373
Tyler CW (1978) Selectivity for spatial-frequency and bar width in cat visual-cortex. Vision Res 18(1): 121–122
VonDer Heydt R (1987) Approaches to visual cortical function. Rev Physiol Biochem Pharmacol 108: 69–150
Xu XM, Ichida JM, Allison JD, Boyd JD, Bonds AB, Casagrande V (2001) A comparison of koniocellular, magnocellular and parvocellular receptive field properties in the lateral geniculate nucleus of the owl monkey (Aotus trivirgatus). J Physiol 531(1): 203–218
Xu XM, Bonds AB, Casagrande VA (2002) Modeling receptive-field structure of koniocellular, magnocellular, and parvocellular LGN cells in the owl monkey (Aotus trivigatus). Visual Neurosci 19(6): 703–711
Zhang YJ (1996) A survey on evaluation methods for image segmentation. Pattern Recogn 29(8): 1335–1346
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Azzopardi, G., Petkov, N. A CORF computational model of a simple cell that relies on LGN input outperforms the Gabor function model. Biol Cybern 106, 177–189 (2012). https://doi.org/10.1007/s00422-012-0486-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-012-0486-6