Abstract
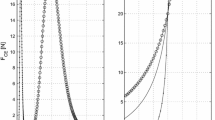
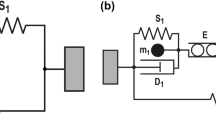
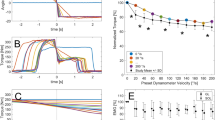
An improved model of locust skeletal muscle will inform on the general behaviour of invertebrate and mammalian muscle with the eventual aim of improving biomedical models of human muscles, embracing prosthetic construction and muscle therapy. In this article, the isometric response of the locust hind leg extensor muscle to input pulse trains is investigated. Experimental data was collected by stimulating the muscle directly and measuring the force at the tibia. The responses to constant frequency stimulus trains of various frequencies and number of pulses were decomposed into the response to each individual stimulus. Each individual pulse response was then fitted to a model, it being assumed that the response to each pulse could be approximated as an impulse response and was linear, no assumption were made about the model order. When the interpulse frequency (IPF) was low and the number of pulses in the train small, a second-order model provided a good fit to each pulse. For moderate IPF or for long pulse trains a linear third-order model provided a better fit to the response to each pulse. The fit using a second-order model deteriorated with increasing IPF. When the input comprised higher IPFs with a large number of pulses the assumptions that the response was linear could not be confirmed. A generalised model is also presented. This model is second-order, and contains two nonlinear terms. The model is able to capture the force response to a range of inputs. This includes cases where the input comprised of higher frequency pulse trains and the assumption of quasi-linear behaviour could not be confirmed.
Similar content being viewed by others
References
Ahn AN, Full RJ (2002) A motor and a brake: two leg extensor muscles acting at the same joint manage energy differently in a running insect. J Exp Biol 205: 379–389
Bakshi UA, Bakshi VU (2007) Control Engineering, chap 3. Published Technical Publications Pune, Perth
Bawa P, Mannard A, Stein RB (1976a) Effects of elastic loads on the contractions of cat muscles. Biol Cybern 22(3): 129–137
Bawa P, Mannard A, Stein RB (1976b) Predictions and experimental tests of a visco-elastic muscle model using elastic and inertial loads. Biol Cybern 22(3): 139–145
Bennet-Clark HC (1975) The energetics of the jump of the locust schistocerca gregaria. J Exp Biol 63: 53–83
Bobet J, Stein RB (1998) A simple model of force generation by skeletal muscle during dynamic isometric contractions. IEEE Trans Biomed Eng 45(8): 1010–1016
Bobet J, Stein RB, Oguztoreli MN (1993) A linear time—varying model of force generation in skeletal muscle. IEEE Trans Biomed Eng 40(10): 1000–1006
Bobet J, Gossen ER, Stein RB (2005) A comparison of models of force production during stimulated isometric ankle dorsiflexion in humans. IEEE Trans Neural Syst Rehabil Eng 13(4): 444–451
Burns MD, Usherwood PNR (1979) Control of walking in orthoptera. 2. Motor neuron activity in normal free-walking animals. J Exp Biol 79: 69–98
Burrows M (1996) The neurobiology of an insect brain. Oxford University Press, New York
Burrows M, Horridge GA (1974) The organization of inputs to motoneurons of the locust metathoracic leg. Philos Trans R Soc Lond B Biol Sci 269: 49–94
Burrows M, Morris G (2001) The kinematics and neural control of high-speed kicking movements in the locust. J Exp Biol 204: 3471–3481
Celichowski J, Raikova R, Drzymala-Celichowska H, Ciechanowicz-Kowalczyk I, Krutki P, Rusev R (2008) Model-generated decomposition of unfused tetani of motor unites evoked by random stimulation. J Biomech 41: 3448–3454
Ding J, Wexler AS, Binder-Macleod SA (2002) A mathematical model that predicts the force-frequency relationship of human skeletal muscle. Muscle Nerve 26(4): 477–485
Ding J, Lee SC, Johnston TE, Wexler WB, AS and Scott and Binder-Macleod SA (2005) Mathematical model that predicts isometric muscle forces for individuals with spinal cord injuries. Muscle Nerve 31(6):702–712
Ebashi S (1980) The croonian lecture, 1979: regulation of muscle contraction. Proc R Soc Lond B Biol Sci 207: 259–286
Gabriel JM (1985) The development of the locust jumping mechanism. II. Energy storage and muscle mechanics. J Exp Biol 118: 327–340
Ghigliazza R, Holmes P (2005) Towards a neuromechanical model for insect locomotion: Hybrid dynamical systems. Regul Chaotic Dyn 10(2): 193–225
Guschlbauer C, Scharstein H, Buschges A (2007) The extensor tibiae muscle of the stick insect: biomechanical properties of an insect walking leg muscle. J Exp Biol 210: 1092–1108
Heitler WJ (1974) Locust jump—specializations of metathoracic femoral-tibial joint. J Comp Physiol 89(1): 93–104
Heitler WJ (1977) The locust jump. III. Structural specializations of the metathoracic tibiae. J Exp Biol 67: 29–36
Heitler WJ (1988) The role of the fast extensor motor activity in the locust kick reconsidered. J Exp Biol 136: 289–309
Heitler WJ, Burrows M (1977) The locust jump. I. The motor programme. J Exp Biol 66: 203–219
Hoyle G (1955a) The anatomy and innervation of locust skeletal muscle. Proc R Soc Lond B 143: 281–292
Hoyle G (1955b) Neuromuscular mechanisms of a locust skeletal muscle. Proc R Soc Lond B 143: 343–367
Hoyle G (1978) Distributions of nerve and muscle fibre types in locust jumping muscle. J Exp Biol 73: 205–233
Huxley AF (1974) Muscular contraction. J Physiol 243(1): 1–43
Law LAF, Shields RK (2005) Mathematical models use varying parameter strategies to represent paralyzed muscle force properties: a sensitivity analysis. J Neuroeng Rehabil 2(12): 18
Mannard A, Stein B (1973) Determination of the frequency response of isometric soleus muscle in the cat using random nerve stimulation. J Physiol 229: 275–296
Parmiggiani F, Stein RB (1981) Nonlinear summation of contractions in cat muscles. II. Later facilitation and stiffness changes. J Gen Physiol 78: 295–311
Raikova R, Celichowski J, Pogrzebna M, Aladjov H, Krutki P (2007) Modeling of summation of individual twitches into unfused tetanus for various types of rat motor units. J Electromyogr Kinesiol 17(2): 121–130
Raikova R, Pogrzebna M, Drzymala H, Celichowski J, Aladjov H (2008) Variability of successive contractions subtracted from unfused tetanus of fast and slow motor units. J Electromyogr Kinesiol 18(5): 741–751
Stein RB, Parmiggiani F (1981) Nonlinear summation of contractions in cat muscles. I. Early depression. J Gen Physiol 78(3): 277–293
The MathWorks Inc. (2001) Curve fitting toolbox user’s guide, (Version 1 ed.). The MathWorks Inc., Natick
Theophilidis G, Burns MD (1983) The innervation of the mesothoracic flexor tibiae muscle of the locust. J Exp Biol 105: 373–388
Van Zandwijk J, Bobbet M, Baan G, Huijing A (1996) From twitch to tetanus: performance of excitation dynamics optimised for a twitch in predicting tetanic muscle forces. Biol Cybern 75: 409–417
Wexler AS, Ding J, Binder-Macleod SA (1997) A mathematical model that predicts skeletal muscle force. IEEE Trans Biomed Eng 44(5): 337–348
Wilson E, Rustighi E, Mace ER, Newland PL (2010) Isometric force generated by locust skeletal muscle: responses to single stimuli. Biol Cybern 102(6): 503–511
Zajac FE (1989) Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng 17(4): 359–411
Zakotnik J (2006) Biomechanics and neural control of targeted limb movements in an insect. Ph.D. thesis, University of Bielfield
Zakotnik J, Matheson T, Durr V (2006) Co-contraction and passive forces facilitate load compensation of aimed limb movements. J Neurosci 26: 4995–5007
Zhou BH, Solomonow M, Baratta R, D’Ambrosia R (1995) Dynamic performance model of an isometric muscle-joint unit. Med Eng Phys 17(2): 145–150
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilson, E., Rustighi, E., Mace, B.R. et al. Modelling the isometric force response to multiple pulse stimuli in locust skeletal muscle. Biol Cybern 104, 121–136 (2011). https://doi.org/10.1007/s00422-011-0423-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-011-0423-0