Abstract
Purpose
This study assessed the effects of upper-body rowing exercise on cardiorespiratory fitness, traditional cardiometabolic risk factors, and vascular health in individuals with spinal cord injury (SCI).
Methods
Seventeen male and female adults with chronic (> 1 yr) motor-complete and incomplete SCI (level of injury: C4-L3) were randomized to control (CON, n = 9) or exercise (UBROW, n = 8). Participants in UBROW performed 12-week, 3 weekly sessions of 30-min upper-body ergometer rowing exercise, complying with current exercise guidelines for SCI. Cardiorespiratory fitness (\(\dot{\rm V}\)O2peak), traditional risk factors (lipid profile, glycemic control) as well as inflammatory and vascular endothelium-derived biomarkers (derived from fasting blood samples) were measured before and after 6 (6W) and 12 weeks (12W). Brachial artery resting diameter and flow-mediated dilation (FMD) were determined by ultrasound as exploratory outcomes.
Results
UBROW increased \(\dot{\rm V}\)O2peak from baseline (15.1 ± 5.1 mL/kg/min; mean ± SD) to 6W (16.5 ± 5.3; P < 0.01) and 12W (17.5 ± 6.1; P < 0.01). UBROW increased resting brachial artery diameter from baseline (4.80 ± 0.72 mm) to 12W (5.08 ± 0.91; P < 0.01), with no changes at 6W (4.96 ± 0.91), and no changes in CON. There were no significant time-by-group interactions in traditional cardiometabolic blood biomarkers, or in unadjusted or baseline diameter corrected FMD. Explorative analyses revealed inverse correlations between changes (∆12W-baseline) in endothelin-1 and changes in resting diameter (r = − 0.56) and FMD% (r = − 0.60), both P < 0.05.
Conclusion
These results demonstrate that 12 weeks of upper-body rowing complying with current exercise guidelines for SCI improves cardiorespiratory fitness and increases resting brachial artery diameter. In contrast, the exercise intervention had no or only modest effects on traditional cardiometabolic risk factors. The study was registered at Clinicaltrials.gov (N-20190053, May 15, 2020).
Similar content being viewed by others
Introduction
Evidence from large epidemiological studies suggests that individuals with spinal cord injury (SCI) are at an approximately two- to threefold higher risk of type 2 diabetes mellitus (Cragg et al. 2013a) and atherosclerotic cardiovascular disease (CVD) (Cragg et al. 2013b), collectively referred to as cardiometabolic diseases. Compared with able-bodied individuals, several studies have reported an elevated prevalence of ‘traditional’ risk factors associated with cardiometabolic diseases in SCI, including reduced high-density lipoprotein cholesterol (HDL-C) (Gilbert et al. 2014), central adiposity (Edwards et al. 2008), and insulin resistance (Bauman and Spungen 1994), a hallmark of the metabolic syndrome (Grundy et al. 2005). However, not all studies show higher prevalence of traditional risk factors such as elevated arterial blood pressure, triglycerides (TG), fasting glucose, and low-density lipoprotein cholesterol (LDL-C) in individuals with SCI, compared with able-bodied (Krum et al. 1992; Liang et al. 2007; Finnie et al. 2008). These findings are supported by a recent study demonstrating that CVD risk, estimated via the Framingham risk score, underestimates true, 5-year occurrence of CVD events in SCI (Barton et al. 2021). Other risk factors than those traditionally associated with cardiometabolic diseases may therefore contribute to the exaggerated CVD risk observed in individuals with SCI including cardiorespiratory fitness, vascular function, and novel biomarkers of cardiovascular risk.
It is well established that regular whole-body exercise reduces the risk for atherosclerotic CVD in able-bodied (Thompson et al. 2003), partly mediated through reduction in traditional cardiometabolic risk factors (Mora et al. 2007). Benefits of exercise may also be related to improvements in cardiorespiratory fitness and novel biomarkers of cardiovascular risk (Green et al. 2017). Furthermore, hemodynamic stimuli during exercise evoke anti-atherogenic adaptations in the vasculature, providing a plausible contribution to some of the unexplained CVD risk reduction with exercise (Green et al. 2013a). A 16-week randomized controlled trial, adopting the 2011 exercise guidelines for adults with SCI (Ginis et al. 2011) (i.e. 2 × 20-min/week moderate-to-vigorous intensity aerobic exercise plus muscle strengthening), was effective in improving cardiorespiratory fitness and muscle strength (Pelletier et al. 2015). However, Zepetnek et al. (2015) demonstrated that this same exercise paradigm was insufficient for improving traditional cardiometabolic risk factors and vascular health (including brachial artery flow-mediated dilation, FMD). In 2018, exercise guidelines for adults with SCI was updated (Ginis et al. 2011, 2018), proposing that a larger volume of moderate-to-vigorous intensity aerobic exercise (i.e., ≥ 30-min, 3 days/week) are required for improvement in cardiometabolic risk factors. However, to date, there is limited knowledge about the efficacy of these new exercise guidelines on cardiometabolic health and vascular adaptations.
Upper-body aerobic exercise is typically performed using a limited number of exercise modalities such as arm-cranking, handcycling, or wheelchair ergometry/propulsion (Eitivipart et al. 2019). Recently, upper-body (i.e., arms-only) ergometer rowing adapted for wheelchair users has shown to be a feasible and acceptable exercise modality that is able to evoke moderate-to-vigorous intensity exercise for individuals with SCI (Sawatzky et al. 2022; Hansen et al. 2022). The primary aims of this study were to assess the impact of 12-week upper-body ergometer rowing (following the 2018 exercise guidelines for individuals with SCI) on cardiorespiratory fitness and traditional cardiometabolic risk factors (fasting insulin and glucose, lipid profile, blood pressure, body mass, and visceral obesity). Additionally, we explored effects of the exercise intervention on upper-extremity conduit artery adaptations by measuring brachial artery resting diameter and FMD. To anticipate for potential time-dependent adaptations, we evaluated the effects on all outcomes after 6 and 12 weeks of exercise. We hypothesized that upper-body rowing exercise would improve cardiorespiratory fitness, and biomarkers of cardiometabolic health.
Methods
This randomized controlled study was approved by the Ethics Committee of North Denmark (N-20190053) and conducted in accordance with the Declaration of Helsinki. After having received oral and written information about the study, all participants provided written informed consent.
Participants
Community-dwelling participants eligible for inclusion had to have a chronic (≥ 1 year since injury) traumatic or non-traumatic SCI; having sufficient arm flexor function to participate in upper-body rowing; and using a manual wheelchair for mobility (≥ 75% of the waking day). Both males and females were included. Potential participants were excluded if they had received a cortisone injection in the shoulder within the last 4 months; known medical issues (urinary tract infections and pressure sores at the time of enrollment, cardiovascular contraindications for exercise testing); diagnosed diabetes or any disease that restricted the ability to exercise.
Neurological level of injury (NLI), and American Spinal Injury Association Impairment Scale (AIS) classification were assessed according to the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) (Kirshblum et al. 2011).
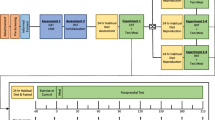
After baseline laboratory assessment, and consistent with the study protocol proposing to include 30 participants, participants were stratified based on sex, age (≥ vs. < 50 years), level of injury (cervical vs. thoracic, lumbar) and baseline cardiorespiratory fitness level (i.e., peak rate of oxygen consumption (\(\dot{\rm V}\)O2peak) > vs. ≤ 16 mL/kg/min), and then randomly assigned to either control (CON) or upper-body exercise training performed on a rowing ergometer adapted to wheelchair users (UBROW) (Hansen et al. 2020). Importantly, even though the different strata were small, it was possible to randomize participants within each stratum so that the group allocation remained unpredictable. In two cases, we pooled two strata, by eliminating one of the four stratification variables (age), so that we could maintain a strata size of minimum two participants. Participants randomized to CON were asked to maintain their normal lifestyle throughout the 12 weeks.
Exercise training intervention
Participants randomized to UBROW performed three weekly sessions of 30 min of moderate-to-vigorous intensity exercise on a rowing ergometer modified for wheelchair users. The target of 30 min of moderate-to-vigorous intensity was reached through individualized progression of session duration and workload (Tweedy et al. 2017). A commercially available rowing ergometer (Concept 2, RowErg D PM5, Morrisville, Vermont, USA) was adapted to wheelchair users by using an Adapt2row unit, as previously described (Hansen et al. 2020).
Details about the ergometer rowing exercise, including how the exercise was adapted to participants with varying injury levels, are reported previously (Hansen et al. 2022). Briefly, while seated in their wheelchair, participants performed dynamic upper-body exercise by repetitively pulling the handle of the rowing ergometer toward the ribcage. This dynamic movement occurred either with or without assistive equipment for stabilization of the torso (i.e., trunk vest and/or Velcro strap), depending on the participants’ level and severity of SCI (Hansen et al. 2022).
Exercise intensity was prescribed based on rating of perceived exertion (RPE), aiming at intensities from moderate (RPE: 12–13) to vigorous (RPE: 14–17) (Riebe et al. 2018) using the Borg 6–20 RPE scale (Borg 1970). The use of RPE to control moderate and vigorous intensity has been validated in individuals with SCI (Goosey-Tolfrey et al. 2010). Additionally, participants were equipped with a heart rate belt (Suunto, iQniter, Aalborg, Denmark) for continuous recording of heart rate. All exercise sessions were supervised by health professionals with knowledge about exercise considerations for individuals with SCI.
Adherence to the intervention (number of sessions participated out of the total 288 sessions (36 sessions per participant) was recorded and presented as adherence rate (% participation).
Main study day protocol
All training sessions and experimental procedures were performed at the sports laboratory, Aalborg University, whereas blood sampling and analyses were conducted at Aalborg University Hospital. The experimental procedures were performed at baseline and after 6 (6W) and 12 weeks (12W). Participants were asked not to change current dietary habits during the 12-week intervention period.
To minimize circadian influence on vascular function, if any (ter Avest et al. 2005), time of day for the experimental procedures were standardized for each participant (± 1 h:39 min). Before each visit, participants were instructed to refrain from a) any vigorous intensity exercise (< 24 h), b) caffeine, polyphenols, alcohol, and vitamin C (< 12 h), and c) food (< 3 h). Participants were encouraged to withdraw from any medication < 24 h before each visit, and if not possible, they were instructed to keep medication intake constant across visits.
Outcomes
At the beginning of the laboratory visit, participants self-reported information about age, smoking status, and for the females, their current phase of the menstrual cycle, if premenopausal.
Resting systolic and diastolic blood pressure
After at least 10 min of rest, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured with the participants seated in their wheelchair using an automated monitoring device (OMRON M3, OMRON Healthcare, Hoofddorp, The Netherlands) (Hansen et al. 2020).
Anthropometric measurements
Using a platform wheelchair scale (KERN EOB 300K100L, Balingen, Germany), body mass was measured with the participant seated in their wheelchair and wearing light clothing. Body mass was then calculated by subtracting the mass of the wheelchair from the total mass of the wheelchair and participant. Waist circumference (WC) was measured in duplicate with non-elastic tape applied directly on the skin immediately below the lowest rib following a normal expiration, with participants in the supine position and arms by the side (Hansen et al. 2020). The same investigator performed all measurement, blinded to the results obtained at the earlier visits.
Brachial artery resting diameter and FMD
Brachial artery FMD was assessed as recommended (Thijssen et al. 2019), with all measurements performed by the same experienced operator.
Participants rested in the supine position for 10–15 min in a quiet, temperature-controlled room (range: 22.1–24.2 °C) before brachial artery examination was performed with the participant’s right arm extended and comfortably positioned at an angle of ~ 80° from the torso on an adjustable table. A rapid inflation and deflation (5 cm) pneumatic cuff (E20. Hokanson Inc., Bellevue, WA, USA) was placed on the right forearm immediately distal to the olecranon process to provide a stimulus to forearm ischaemia.
A 10 MHz multifrequency linear array ultrasound probe coupled to a high-resolution ultrasound machine (LOGIQ S8 XDclear, GE Healthcare) was used to insonate the brachial artery in the distal one-third of the upper arm, with the ultrasound parameters set to optimize the longitudinal, B-mode images of the arterial wall-lumen interface. Baseline diameter was recorded for ≥ 30 s prior to inflation of the pneumatic cuff. The cuff was then inflated for 5 min to ≥ 200 mmHg (at least 50 mmHg > systolic pressure to prevent arterial inflow). Immediately prior to deflation, diameter recordings resumed and continued for additional 3 min following cuff deflation. Insonation settings and occlusion pressures were kept constant within participants across study visits.
Brachial artery lumen diameter was analyzed offline frame-by-frame using semi-automated edge-detecting and wall-tracking software (Medical Imaging Applications, LLC, Coralville, IA, USA), which is largely independent of investigator bias. Peak diameter following cuff release was automatically detected based on a 3-s rolling average. Baseline resting diameter was calculated as the mean of the 30-s resting period. FMD was calculated as the absolute (mm) and relative (%) difference between peak and baseline diameter. In addition, an allometrically scaled FMD was calculated to adjust for differences in resting baseline diameter (corrected FMD%) (Atkinson and Batterham 2013).
Cardiorespiratory fitness
Participants performed a graded exercise test to exhaustion (GTX) on an arm-crank ergometer (Monark 881E, Vansbro, Sweden) for determination of \(\dot{\rm V}\)O2peak using an open-circuit metabolic cart (JAEGER, Vyntus CPX, Carefusion), as described in detail in the study protocol (Hansen et al. 2020). After a brief warm-up with zero resistance, the test began at an individualized starting load (0–50 W) performed for 1 min, followed by an increase in workload every min (3.5 or 7 W/min) until volitional exhaustion (Eerden et al. 2018). Starting load and magnitude of increment were kept constant within participants across study visits.
Heart rate and breath-by-breath \(\dot{\rm V}\)O2, carbon dioxide production and minute ventilation were measured continuously throughout the test, with RPE noted at the end of each min. Gas exchange parameters were exported for offline analysis, with the highest 30 s average reported as \(\dot{\rm V}\)O2peak and the highest power output that could be sustained for ≥ 30 s reported as POpeak (Hansen et al. 2020).
Cardiometabolic blood biomarkers
After an overnight fast (≥ 10 h), approximately 50 mL of blood was drawn from the antecubital vein. Blood samples were aliquoted and stored at − 80 °C until analyses. For some participants, blood sampling was performed on a separate day than the university laboratory visit (with a maximum of 4 days in between blood sampling and laboratory visit), whereas for others, blood sampling was performed immediately before arriving at the laboratory. Notably, each participants used the same procedure for all three (baseline, 6W, 12W) visits. For participants allocated to UBROW, blood sampling was performed between 36 and 60 h after the last exercise session (both at 6W and 12W) to reduce any acute effects from the last exercise session on blood biomarkers.
Fasting levels of total cholesterol, HDL-C, TG, C-reactive protein (CRP), glucose, insulin, and glycated hemoglobin (HbA1c) were measured in a routine hospital laboratory accredited according to the DS/EN ISO 15189, and LDL-C was calculated using the Friedewaldt equation. The homeostasis model assessment 2 of insulin resistance (HOMA2-IR), pancreatic β-cell function (HOMA2-β), and insulin sensitivity (HOMA2-S) were calculated using the HOMA2 calculator https://www.dtu.ox.ac.uk/homacalculator/.
Levels of tumor necrosis factor alpha (TNF-α) (V-PLEX kit K151A9H-1/K0081839) as well as soluble intercellular adhesion molecule-1 (sICAM-1) and soluble vascular cell adhesion molecule-1 (sVCAM-1) (V-PLEX kit K151A9H-1/K0081821) were measured in plasma samples using chemiluminescence-based assays from Meso Scale Discovery (MSD, Gaithersburg, MD, USA) on EDTA plasma. Samples were run in singlets in two experiments, and analysis was done using a QuickPlex SQ 120MM instrument (MSD) and the DISCOVERY WORKBENCH® 4.0 software.
Endothelin-1 (ET-1) was analyzed using the Quantikine ELISA Endothelin-1 immune-assay (R&D, Abingdon, UK). EDTA plasma samples were run undiluted in singlets essentially as described by the manufacturer. Assay controls were run in three levels (R&D, Abingdon, UK). According to the manufacturer, intra-assay CVs are 1.9–4% and inter-assay CVs are 5.3–7.6%. In our laboratory, we found inter-assay CVs of 1.5–3.5% (low and medium controls).
Statistics
Linear mixed model analyses were used to determine main effects of ‘time’ (baseline, 6W and 12W) and ‘time-by-group’ interactions. Time, group, and the interaction were used as fixed factors for the model. Bonferroni-corrected post hoc tests were performed to account for multiple comparisons where appropriate. For the allometrically scaled FMD, the logarithmically transformed baseline and peak diameters were used to derive the diameter changes on the logged scale, which then were entered into a two-way ANCOVA, with ‘time’ and ‘group’, as fixed factors, and the logarithmically transformed baseline diameter as covariate. Corrected FMD values and SD were then back calculated from the estimated means and SE values, as recommended (Atkinson and Batterham 2013). Normal distribution of participant characteristics (continuous data) was tested with the Shapiro–Wilk test. Chi-square, and independent t-tests (or Mann–Whitney U tests for non-normal data) were performed to determine group differences in categorical and continuous baseline characteristics, respectively. Standardized effect sizes (Hedges’s g) for absolute change scores (12W-baseline) were calculated to determine the magnitude of difference in responses between groups, with the following thresholds: trivial (< 0.2), small (> 0.2), moderate (> 0.5), and large (> 0.8). Positive values of Hedges’s g indicate a favor of UBROW, while negative values suggest a favor of CON. Pearson’s correlations were used to explore potential associations between changes (from baseline to 12 W) in circulatory endothelium-derived (sICAM-1, sVCAM-1, ET-1) and inflammatory (TNF-α, CRP) markers, and changes (from baseline to 12 W) in resting lumen diameter and FMD.
Statistical analyses were performed using SPSS (version 27; IBM, Armonk, New York). Statistical significance was accepted at α < 0.05. In text and tables, values are means with SD, unless otherwise stated, with effects from baseline to 12W expressed as change scores (∆) and 95% confidence intervals (CI).
Results
This study was conducted during the COVID-19 pandemic, with national restrictions limiting the number of participants we were able to include. Accordingly, 15 out of 17 included participants at baseline completed the study (Fig. 1) and were characterized as shown in Table 1. All participants in UBROW (n = 8) completed the study, with an exercise session adherence rate of 92 ± 13%, with four out of eight participants completing all 36 sessions. Over the 12 weeks, the eight participants rowed an average of 28:42 ± 02:07 min per session, with an average distance of 4.1 ± 1.2 km per session. Power output and RPE averaged 42 ± 21 W and 15.8 ± 0.7, respectively, across all sessions. The average heart rate during the exercise (n = 7) reached 130 ± 12 bpm, corresponding to 83 ± 3% of participant’s individual peak heart rate (HRpeak). For one participant (AIS: B, NLI: C6), heart rate did not increase above 100–115 bpm during either the GTX or the first rowing sessions. Thus, exercise intensity was solely quantified based on RPE for this participant.
There were no differences between groups at baseline (P ≥ 0.079; Table 1). There were no smokers in any of the groups, and all female participants were menopausal or postmenopausal at the time of enrollment.
\(\dot{\rm V}\) O 2peak and PO peak
For absolute \(\dot{\rm V}\)O2peak (P < 0.001) and relative \(\dot{\rm V}\)O2peak (P < 0.001), we found significant time-by-group interactions. Post hoc tests revealed that absolute \(\dot{\rm V}\)O2peak increased from baseline to 6W (P = 0.011) and 12W (P < 0.001; ∆186 mL/min; 95% CI 79–292 mL/min; g = 2.04) in UBROW, with no changes in CON. Relative \(\dot{\rm V}\)O2peak increased from baseline to 6W (P < 0.001) and 12W (P < 0.001; ∆2.4 mL/kg/min; 95% CI 0.9–3.9 mL/kg/min; g = 2.03) in UBROW, whereas CON had a significant reduction from baseline to 6W (P = 0.041), but not at 12W (Fig. 2a, b). There was a significant time-by-group interaction for POpeak (P < 0.001). Post hoc tests showed that POpeak increased from baseline to 6W (P < 0.001) and 12W (P < 0.001; ∆17 W; 95% CI 10–24 W; g = 2.44) in UBROW, with no changes in CON (Fig. 2c, d).
Anthropometrics and arterial blood pressure
Body mass, SBP, or DBP revealed no time-by-group interactions or main effect of time (Table 3). There was a tendency for a time-by-group interaction (P = 0.061) for WC, with no main effect of time (Table 3).
Blood biomarkers
For sICAM-1, we found a significant main effect of time (P < 0.001), with no time-by-group interaction (Table 2), whereas ET-1 levels revealed a tendency for a time-by-group interaction (P = 0.078), with no main effect of time. There were no time-by-group interactions or main effects of time for the other vascular injury or inflammatory risk markers (sVCAM-1, CRP, TNF-α) (Table 2). HOMA2-β levels showed a significant time-by-group interaction (P = 0.04) (Table 3), and post hoc tests revealed that HOMA2-β increased from baseline to 6W (P = 0.012) and 12W (P = 0.017) in CON, with no changes in UBROW. There was a significant main effect of time for fasting glucose (P = 0.018), with no time-by-group interaction, while no time-by-group interactions or main effects of time were observed for the other cardiometabolic risk markers (Table 3). Due to a missing blood sample from one participant, results of some biomarkers (Table 3) are from n = 6 in CON.
Brachial artery resting diameter and FMD
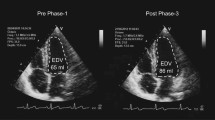
Due to technical issues with the ultrasound machine, there are missing recordings of post-deflation diameter (and thus FMD) at baseline for two participants in CON and one participant in UBROW (Fig. 3 and Table 2). There was a significant time-by-group interaction for resting brachial artery lumen diameter (P = 0.016). Post hoc tests revealed increased resting diameter from baseline to 12W (P = 0.005; ∆0.28 mm; 95% CI 0.05–0.53 mm; g = 1.34) in UBROW, with no changes at 6W, and no changes in CON, Fig. 3a, b. There were no time-by-group interactions or main effects of time for absolute (Table 2) and relative FMD (Fig. 3c, d), or corrected FMD% (Table 2).
Group mean and individual data on resting brachial artery lumen diameter and the unadjusted relative change in brachial artery diameter from baseline (FMD%) in response to 5 min ischemia in CON (a, c) and UBROW (b, d), at baseline (BL) and at 6W and 12W. #Significantly different from baseline (P ≤ 0.01). Note that for FMD%, n = 5 in CON and n = 7 in UBROW
Associations between resting diameter and FMD, and inflammatory and vascular endothelium-derived blood biomarkers
We found a significant inverse correlation (r = − 0.56, P = 0.036, n = 14) between the changes in ET-1 from baseline to 12W (∆12W-baseline) and the changes (∆12W-baseline) in resting brachial artery lumen diameter. There was a significant, inverse correlation (r = − 0.60, P = 0.049, n = 11) between the ∆12W-baseline in ET-1 and the ∆12W-baseline in FMD%. There were no correlations between the changes in resting lumen diameter or FMD% and the changes in sICAM-1, sVCAM-1, TNF-α, and CRP (P ≥ 0.238).
Discussion
In this study, we demonstrate that 12 weeks of upper-body ergometer rowing exercise increases cardiorespiratory fitness and resting brachial artery lumen diameter, with no, or only modest, effects on traditional cardiometabolic risk factors. Notably, whilst aerobic fitness (POpeak and \(\dot{\rm V}\)O2peak) improved within the first 6 weeks, our findings also indicate that 12 weeks of moderate-to-vigorous intensity upper-body rowing exercise, complying with the current exercise guidelines, are required for increasing diameter of the brachial artery supplying the active limbs of wheelchair users with SCI.
Cardiorespiratory fitness
The increase in aerobic fitness, as reflected by enhanced \(\dot{\rm V}\)O2peak and POpeak, in UBROW aligns with the current evidence suggesting that upper-body exercise training improves aerobic fitness level in individuals with SCI (Eitivipart et al. 2019). At 12W, \(\dot{\rm V}\)O2peak and POpeak were increased relative to baseline in UBROW by 16 and 27%, respectively. Such improvements in aerobic fitness are larger than reported (~ 10%) by other studies, in individuals with SCI, using a similar duration (12 weeks) and weekly volume (90 min) of moderate-to-vigorous intensity arm-cranking exercise (Rosety-Rodriguez et al. 2014; El-Sayed and Younesian 2005). Possibly, the involvement of a relatively large muscle mass of the upper-body when rowing (Troy 2011) may have imposed a cardiovascular challenge that stimulated greater adaptations in cardiorespiratory fitness. As a novel contribution, we demonstrated an approximately 10% improvement in \(\dot{\rm V}\)O2peak within the first 6 weeks, which provide new information about the time-dependent adaptations in cardiorespiratory fitness among individuals with SCI. Although cardiorespiratory fitness is not considered a traditional risk factor for cardiometabolic diseases (Wilson et al. 1998; Grundy et al. 2005), there is a strong inverse association between \(\dot{\rm V}\)O2peak and all-cause mortality in able-bodied (Blair et al. 1989). Therefore, the improvements in \(\dot{\rm V}\)O2peak in this study may have led to substantial CVD risk reduction that was not accounted for via the traditional risk factors. According to the principle of specific adaptations to imposed demands, improvement in \(\dot{\rm V}\)O2peak would likely have exceeded the reported 16% increase if the GTX had been performed on the rowing ergometer. However, the arm-crank ergometer (unspecific exercise modality) was chosen for the GTX to ensure that both groups were equally familiarized with the test modality. This approach allows us to interpret the increases in POpeak and \(\dot{\rm V}\)O2peak in UBROW as improvements in exercise capacity and cardiorespiratory fitness, respectively, due to physiological adaptations, and not merely due to a ‘learning’ effect.
Traditional cardiometabolic risk factors
Consistent with the existing literature (Farrow et al. 2020; Nightingale et al. 2017a; Zepetnek et al. 2015), we did not observe any improvements in traditional cardiometabolic blood biomarkers (e.g., lipid profile, glycemic control), body mass, or resting arterial blood pressure following the 12-week exercise intervention. Due to the lack of involvement of the large muscle groups in the lower extremities when performing isolated upper-body exercise, it is possible that the absolute energy expenditure during the exercise sessions was insufficient in creating a negative energy balance eliciting meaningful reductions in body mass (Donnelly et al. 2009) and blood biomarkers, such as lipid profile (Mann et al. 2014). One solution could be to raise the rate of energy expenditure (i.e., exercise intensity) (Nightingale et al. 2017b) compared with the currently recommended ‘moderate-to-vigorous’ intensity (Ginis et al. 2018). However, the average RPE and %HRpeak across the 12 weeks confirmed that participants in this study exercised with ‘vigorous’ intensity according to SCI-specific intensity classifications (Hutchinson and Goosey-Tolfrey 2022). Hence, a longer duration and/or larger volume of moderate-to-vigorous intensity exercise than recommended (Ginis et al. 2018) may be required for providing a sufficient stimulus for meaningful changes in traditional risk factors. Alternatively, the lack of effect of upper-body rowing on traditional risk factors may simply be a consequence of the relatively healthy cohort participating in this study. According to criteria of The American Heart Association (AHA)/National Heart, Lung, and Blood Institute (NHLBI) (Grundy et al. 2005), and SCI-specific criteria for abdominal obesity (WC) (Ravensbergen et al. 2014), only two of our participants (both from UBROW) met the clinically classification for the metabolic syndrome at baseline, thereby limiting the ‘window’ for improvement. Future studies may consider including a homogenous group of participants with the metabolic syndrome to elucidate the potential of upper-body rowing on lowering cardiometabolic risk in this population.
Notwithstanding the lack of effects of upper-body rowing on traditional risk factors, we did, however, find a tendency for a time-by-group interaction for the cardiometabolic risk factor, WC. The absolute change in WC (∆ − 5.7 cm) and large effect size (g = − 0.86) indicate that upper-body rowing evoked a clinically relevant reduction in abdominal adiposity. This finding is consistent with current evidence suggesting that upper-body aerobic exercise elicits reductions in WC (Farrow et al. 2020). Considering the clinically relevant reduction in abdominal adiposity, despite only a modest reduction in body mass (∆ − 1.2 kg, g = − 0.59), could indicate that upper-body rowing may have led to changes in body composition through accretion in lean mass. However, direct measurements of body composition via dual energy X-ray absorptiometry scans or magnetic resonance imaging are required to test this hypothesis.
Brachial artery resting diameter and FMD
Cross-sectional studies in wheelchair athletes and able-bodied controls have provided indirect evidence suggesting that regularly performed upper-body exercise is associated with localized increases in resting conduit artery diameter of the upper-extremities (Rowley et al. 2012; Huonker et al. 2013; Stoner et al. 2006). Complying with the previous exercise guidelines (2011) for individuals with SCI, Zepetnek (2015) reported that 16 weeks of 40 min/week of moderate-to-vigorous intensity aerobic exercise did not induce changes in brachial artery function (FMD) or structure (resting diameter). Results from the current study therefore extends the existing literature by demonstrating that 12 weeks of upper-body rowing exercise, complying with the current (2018) guidelines, increases resting brachial artery diameter.
Taken together, these results provide evidence for a critical role of exercise dose for vascular adaptations and imply that 90 min of moderate-to-vigorous intensity upper-body exercise weekly provides an adequate exercise stimulus for adaptations in the vasculature of the active upper-limbs in individuals with SCI.
A strength of this study is that we assessed the effects of the exercise intervention after 6 weeks allowing us to describe the time course of adaptations. This may be particularly important for vascular adaptations, as changes in vascular function may be rapid and short-lived (Green et al. 2017). However, we did not observe changes in vascular function at either 6W or 12W, neither when expressed as unadjusted FMD or when corrected for the potentially confounding influence of resting diameter (corrected FMD%). As initial improvements in vascular dilator function is thought to precede structural adaptations (Laughlin 1995), it raises the question whether early increases in FMD may have occurred, but remained undetected at the 6W and 12W time points. In support, Tinken et al. (2008) demonstrated different time courses for adaptations to vasodilator function and structure in response to 8 weeks of exercise training in able-bodied. In their study (Tinken et al. 2008), brachial artery FMD increased from baseline to week 2 and week 4, whereas FMD returned toward baseline levels again by week 6 and 8 when structural adaptations started to occur. As we assessed FMD in the already well-conditioned upper-limbs resulting from years of wheelchair use, it may also be that vascular function was already enhanced in our cohort of individuals with SCI, consistent with reports of comparable or even decreased FMD of able-bodied elite athletes, relative to sedentary controls (Green et al. 2013b). Alternatively, the absence of adaptation in brachial artery function may relate to the vigorous intensity performed throughout the intervention. While moderate intensity exercise might confer acute improvements in NO-mediated endothelial function and reductions in oxidative stress (Goto et al. 2003; Dawson et al. 2013), high intensity exercise has been associated with heightened levels of reactive oxygen species (Johnson et al. 2012; Goto et al. 2007) that potentially may blunt some of the vascular benefits of exercise, including FMD (Green et al. 2017; Dawson et al. 2013). Such an effect of vigorous intensity exercise on the vascular wall may also help explain why upper-body rowing did not elicit improvements in inflammatory or vascular adhesive molecules.
We observed a tendency for the vasoconstrictor ET-1 to increase throughout the intervention in CON. In addition to the effects on vascular tone, ET-1 is implicated in the development of atherosclerosis through stimulation of vascular smooth muscle cell proliferation (Komuro et al. 1988). Notably, exercise training has been shown to decrease plasma levels of ET-1 in healthy able-bodied individuals (Maeda et al. 2001; Nyberg et al. 2014), and oppose the age-related elevation in plasma ET-1 and normalize plasma levels of ET-1 in individuals with essential hypertension (Nyberg et al. 2013). Therefore, attenuation of ET-1 through exercise training may be a candidate to explain part of the cardioprotective effects of exercise that is not accounted for by modifications of traditional risk factors in individuals with SCI.
There were significant, inverse associations between the changes in plasma levels of ET-1, and the changes in FMD and resting diameter. While structural (outward) remodeling of the brachial artery may explain part of the increase in resting diameter, the significant inverse association between changes in resting brachial artery lumen diameter and changes in circulating ET-1 suggests that altered vasoconstrictor activity may also contribute to increased resting brachial artery diameter following exercise in individuals with SCI.
Finally, given that a change in autonomic nervous system function has been proposed as a candidate to explain some of the excess beneficial effects of exercise (Joyner & Green 2009), it is possible that the intervention may have induced additional cardioprotective effects that were not captured in the present study. Future exercise studies in individuals with SCI should therefore consider including an assessment of autonomic nervous system function, such as heart rate variability or baroreflex sensitivity (Joyner and Green 2009), and quantify such neural effects according to varying levels of neurological injury.
Methodological considerations
Increased resting conduit artery diameter has been interpreted as indicative of structural remodeling (Dinenno et al. 2001; Rowley et al. 2012). A limitation of this interpretation is that resting arterial diameter is susceptible to factors that may influence vascular tone, such as sympathetic activity and local endothelium-dependent vasoconstrictors and vasodilators. To minimize the potential influence of some of these confounders, we followed expert recommendations (Thijssen et al. 2019) about standardizing participant preparation (e.g., avoiding caffeine, no prior exercise, and a certain period of rest) and protocol (e.g., cuff position, inflation pressure, and ischemic period). Nevertheless, it is a limitation that we did not include a measure of sympathetic nerve activity, such as the skin sympathetic response to nerve stimulation.
In this study, brachial artery FMD was included as an explorative outcome. Nonetheless, the lack of measurement of red blood cell velocity required for shear rate calculation presents a limitation, as shear rate (or shear stress) represents the stimulus for post-deflation vasodilation (Pyke and Tschakovsky 2007).
To delineate any contribution from endothelium-dependent vasoconstrictors to vascular tone, and thus resting diameter, we measured plasma levels of ET-1 and found a tendency for a time-by-group interaction for ET-1. Accordingly, we cannot exclude that elevated levels of ET-1 may have contributed to increased vascular tone in CON and thus influenced resting artery diameter, which is supported by the inverse association between changes in resting brachial artery lumen diameter and changes in circulating ET-1. Moreover, it is possible that other endothelium-derived constricting factors, such as angiotensin II, may have influenced vascular tone (Thijssen et al. 2008).
The relatively small, heterogeneous group of individuals with SCI presents a limitation, which may have increased the risk for type II errors. Even though there were significant changes in \(\dot{\rm V}\)O2peak, POpeak, and resting brachial artery diameter, it is possible that the study was underpowered to detect changes in outcomes with smaller magnitude of change. However, only trivial to moderate effect sizes were evident between groups for the majority of cardiometabolic blood biomarkers, suggesting that the conclusion likely would not be substantially different with inclusion of additional participants. Despite the relatively small sample size, the inclusion of the control group is a considerable strength, as this allowed discriminating a ‘true effect’ of the intervention from any inherent variability in outcome measures and natural time course changes over 12 weeks in this population.
Despite challenges with logistics of conducting a 12-week, supervised exercise intervention during COVID-19 (e.g., that group training was not allowed), participant retention was high. In fact, 15 out of the 17 participants included at baseline completed the study. Notably, all participants in UBROW completed the study, which is remarkable considering the completion rates reported in previous exercise training studies in this population (Ginis and Hicks 2005). In addition, the exercise adherence rate was high, which constitutes an important finding when evaluating the feasibility of upper-body rowing as an alternative to existing upper-body exercise modalities (arm-cranking, wheelchair propulsion, handcycling).
Conclusions
This randomized controlled study provides new evidence that 12 weeks of upper-body ergometer rowing exercise, complying with the current exercise guidelines, improves cardiorespiratory fitness and increases resting brachial artery diameter, in individuals with SCI. In contrast, there were no, or only modest, effects of the exercise training on risk factors traditionally associated with cardiometabolic diseases. Finally, considering the associations between changes in plasma levels of ET-1 and changes in brachial artery resting diameter and FMD, further studies are needed to investigate the potential cardioprotective role of exercise through attenuation of ET-1 in individuals with SCI.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AIS:
-
American Spinal Injury Association Impairment Scale
- CON:
-
Control
- CRP:
-
C-reactive protein
- CVD:
-
Cardiovascular disease
- DBP:
-
Diastolic blood pressure
- ET-1:
-
Endothelin-1
- FMD:
-
Flow-mediated dilation
- GTX:
-
Graded exercise test to exhaustion
- HbA1c:
-
Glycated hemoglobin
- HDL-C:
-
High-density lipoprotein cholesterol
- HOMA2-β:
-
Homeostasis model assessment 2 of pancreatic β-cell function
- HOMA2-IR:
-
Homeostasis model assessment 2 of insulin resistance
- HOMA2-S:
-
Homeostasis model assessment 2 of insulin sensitivity
- HRpeak :
-
Heart rate peak
- LDL-C:
-
Low-density lipoprotein cholesterol
- NLI:
-
Neurological level of injury
- RPE:
-
Rating of perceived exertion
- POpeak :
-
Peak power output
- SBP:
-
Systolic blood pressure
- SCI:
-
Spinal cord injury
- sICAM-1:
-
Soluble intercellular adhesion molecule-1
- sVCAM-1:
-
Soluble vascular cell adhesion molecule-1
- TG:
-
Triglycerides
- TNF-α:
-
Tumor necrosis factor alpha
- UBROW:
-
Upper-body rowing
- \(\dot{\rm V}\)O2peak :
-
Peak rate of oxygen consumption
- WC:
-
Waist circumference
References
Atkinson G, Batterham AM (2013) Allometric scaling of diameter change in the original flow-mediated dilation protocol. Atherosclerosis 226:425–427. https://doi.org/10.1016/j.atherosclerosis.2012.11.027
Barton TJ, Low DA, Bakker EA, Janssen T, de Groot S, van der Woude L et al (2021) Traditional cardiovascular risk factors strongly underestimate the 5-year occurrence of cardiovascular morbidity and mortality in spinal cord injured individuals. Arch Phys Med Rehabil 102:27–34. https://doi.org/10.1016/j.apmr.2020.07.013
Bauman WA, Spungen AM (1994) Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism 43:749–756. https://doi.org/10.1016/0026-0495(94)90126-0
Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW (1989) Physical fitness and all-cause mortality. A prospective study of health men and women. JAMA 262:2395–2401
Borg G (1970) Perceived exertion as an indicator of somatic stress. Scand J Rehab Med 2–3:92–98
Cragg JJ, Noonan VK, Dvorak M, Krassioukov A, Mancini GBJ, Borisoff JF (2013a) Spinal cord injury and type 2 diabetes. Results from a population health survey. Neurology 81:1864–1868. https://doi.org/10.1212/01.wnl.0000436074.98534.6e
Cragg JJ, Noonan VK, Krassioukov A, Borisoff J (2013b) Cardiovascular disease and spinal cord injury. Results from a national population health survey. Neurology 81:723–728. https://doi.org/10.1212/01.wnl.0000436074.98534.6e
Dawson EA, Green DJ, Cable NT, Thijssen DHJ (2013) Effects of acute exercise on flow-mediated dilatation in healthy humans. J Appl Physiol 115:1589–1598. https://doi.org/10.1152/japplphysiol.00450.2013
Dinenno FA, Tanaka H, Monahan KD, Clevenger CM, Eskurza I, Desouza CA et al (2001) Regular endurance exercise induces expansive arterial remodelling in the trained limbs of healthy men. J Physiol 534:287–295. https://doi.org/10.1111/j.1469-7793.2001.00287.x
Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK (2009) Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 41:459–471. https://doi.org/10.1249/MSS.0b013e3181949333
Edwards LA, Bugaresti JM, Buchholz AC (2008) Visceral adipose tissue and the ratio of visceral to subcutaneous adipose tissue are greater in adults with than in those without spinal cord injury, despite matching waist circumferences. Am J Clin Nutr 87:600–607
Eerden S, Dekker R, Hettinga FJ (2018) Maximal and submaximal aerobic tests for wheelchair-dependent persons with spinal cord injury: a systematic review to summarize and identify useful applications for clinical rehabilitation. Disabil Rehabil 40:497–552. https://doi.org/10.1080/09638288.2017.1287623
Eitivipart AC, De Oliveira CQ, Arora M, Middleton J, Davis GM (2019) Overview of systematic reviews of aerobic fitness and muscle strength training after spinal cord injury. J Neurotrauma 36:2943–2963. https://doi.org/10.1089/neu.2018.6310
El-Sayed MS, Younesian A (2005) Lipid profiles are influenced by arm cranking exercise and training in individuals with spinal cord injury. Spinal Cord 43:299–305. https://doi.org/10.1038/sj.sc.3101698
Farrow M, Nightingale TE, Maher J, McKay CD, Thompson D, Bilzon JLJ (2020) Effect of exercise on cardiometabolic risk factors in adults with chronic spinal cord injury: a systematic review. Arch Phys Med Rehabil 101:2177–2205. https://doi.org/10.1016/j.apmr.2020.04.020
Finnie AK, Buchholz AC, Martin Ginis KA, Latimer AE, Bray SR, Craven C et al (2008) Current coronary heart disease risk assessment tools may underestimate risk in community-dwelling persons with chronic spinal cord injury. Spinal Cord 46:608–615. https://doi.org/10.1038/sc.2008.21
Gilbert O, Croffoot JR, Taylor AJ, Nash M, Schomer K, Groah S (2014) Serum lipid concentrations among persons with spinal cord injury—a systematic review and meta-analysis of the literature. Atherosclerosis 232:305–312. https://doi.org/10.1016/j.atherosclerosis.2013.11.028
Ginis KAM, Hicks AL, Latimer AE, Warburton DER, Bourne C, Ditor DS et al (2011) The development of evidence-informed physical activity guidelines for adults with spinal cord injury. Spinal Cord 49:1088–1096. https://doi.org/10.1038/sc.2011.63
Ginis MJW, Kathleen A, van der Scheer AE, Latimer-Cheung A, Barrow C, Bourne P, Carruthers M, Bernardi DS, Ditor S, Gaudet S, de Groot KC, Hayes AL, Hicks CA, Leicht J, Lexell S, Macaluso PJ, Manns CB, McBride VK, Noonan P, Pomerleau JH, Rimmer RB, Shaw B, Smith KM, Smith JD, Steeves D, Tussler CR, West DL, Wolfe VL, Goosey-Tolfrey (2018) Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord 56(4):308–321. https://doi.org/10.1038/s41393-017-0017-3
Goosey-Tolfrey V, Lenton J, Goddard J, Oldfield V, Tolfrey K, Eston R (2010) Regulating intensity using perceived exertion in spinal cord-injured participants. Med Sci Sports Exerc 42:608–613. https://doi.org/10.1249/MSS.0b013e3181b72cbc
Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K et al (2003) Effect of different intensities of exercise on endothelium-dependent vasodilation in humans. Role of endothelium-dependent nitric oxide and oxidative stress. Circulation 108:530–535. https://doi.org/10.1161/01.CIR.0000080893.55729.28
Goto C, Nishioka K, Umemura T, Jitsuiki D, Sakagutchi A, Kawamura M et al (2007) Acute moderate-intensity exercise induces vasodilation through an increase in nitric oxide bioavailability in humans. Am J Hypertens 20:825–830. https://doi.org/10.1016/j.amjhyper.2007.02.014
Green DJ, Driscoll GO, Joyner MJ, Cable NT (2013a) Exercise and cardiovascular risk reduction: time to update the rationale for exercise ? J Appl Physiol 105:766–768. https://doi.org/10.1152/japplphysiol.01028.2007
Green DJ, Rowley N, Spence A, Carter H, Whyte G, George K et al (2013b) Why isn’t flow-mediated dilation enhanced in athletes? Med Sci Sports Exerc 45:75–82. https://doi.org/10.1249/MSS.0b013e318269affe
Green DJ, Hopman MTE, Padilla J, Laughlin MH, Thijssen DHJ (2017) Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev 97:495–528. https://doi.org/10.1152/physrev.00014.2016
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA et al (2005) Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 112:2735–2752. https://doi.org/10.1161/CIRCULATIONAHA.105.169404
Hansen RK, Samani A, Laessoe U, Handberg A, Larsen RG (2020) Effect of wheelchair-modified rowing exercise on cardiometabolic risk factors in spinal cord injured wheelchair users: protocol for a randomised controlled trial. BMJ Open 10:e040727. https://doi.org/10.1136/bmjopen-2020-040727
Hansen RK, de Wit JLJ, Samani A, Laessoe U, Figlewski K, Larsen RG (2022) Wheelchair-modified ergometer rowing exercise in individuals with spinal cord injury: a feasibility, acceptability, and preliminary efficacy study. Spinal Cord Ser Cases 8:48. https://doi.org/10.1038/s41394-022-00518-6
Huonker M, Schmid A, Schmidt-Trucksäß A, Grathwohl D, Keul J (2013) Size and blood flow of central and peripheral arteries in highly trained able-bodied and disabled athletes. J Appl Physiol 95:685–691. https://doi.org/10.1152/japplphysiol.00710.2001
Hutchinson MJ, Goosey-Tolfrey VL (2022) Rethinking aerobic exercise intensity prescription in adults with spinal cord injury: time to end the use of “moderate to vigorous” intensity ? In Press. https://doi.org/10.1038/s41393-021-00733-2
Johnson BD, Padilla J, Wallace JP (2012) The exercise dose affects oxidative stress and brachial artery flow-mediated dilation in trained men. Eur J Appl Physiol 112:33–42. https://doi.org/10.1007/s00421-011-1946-8
Joyner MJ, Green DJ (2009) Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol 587:5551–5558. https://doi.org/10.1113/jphysiol.2009.179432
Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A et al (2011) International standards for neurological classification of spinal cord injury (Revised 2011). J Spinal Cord Med 34:535–546. https://doi.org/10.1179/107902611X13186000420242
Komuro I, Kurihara H, Sugiyama T, Takaku F, Yazaki Y (1988) Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. FEBS Lett 238:249–252. https://doi.org/10.1016/0014-5793(88)80489-7
Krum H, Howes LG, Brown DJ, Ungar G, Moore P, McNeil JJ et al (1992) Risk factors for cardiovascular disease in chronic spinal cord injury patients. Paraplegia 30:381–388. https://doi.org/10.1038/sc.1992.87
Laughlin MH (1995) Endothelium-mediated control of coronary vascular tone after chronic exercise training. Med Sci Sports Exerc 27:1135–1144
Liang H, Chen D, Wang Y, Rimmer JH, Braunschweig CL (2007) Different risk factor patterns for metabolic syndrome in men with spinal cord injury compared with able-bodied men despite similar prevalence rates. Arch Phys Med Rehabil 88:1198–1204. https://doi.org/10.1016/j.apmr.2007.05.023
Maeda S, Miyauchi T, Kakiyama T, Sugawara J, Iemitsu M, Irukayama-Tomobe Y et al (2001) Effects of exercise training of 8 weeks and detraining on plasma levels of endothelium-derived factors, endothelin-1 and nitric oxide, in healthy young humans. Life Sci 69:1005–1016. https://doi.org/10.1016/S0024-3205(01)01192-4
Mann S, Beedie C, Jimenez A (2014) Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sport Med 44:211–221. https://doi.org/10.1007/s40279-013-0110-5
Martin Ginis KA, Hicks AL (2005) Exercise research issues in the spinal cord injured population. Exerc Sport Sci Rev 33:49–53
Martin Ginis KA, Van Der Scheer JW, Latimer-Cheung AE, Barrow A, Bourne C, Carruthers P et al (2018) Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord 56:308–321. https://doi.org/10.1038/s41393-017-0017-3
Mora S, Cook N, Buring JE, Ridker PM, Lee I-M (2007) Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation 116:2110–2118
Nightingale TE, Walhin JP, Thompson D, Bilzon JLJ (2017a) Impact of exercise on cardiometabolic component risks in spinal cord-injured humans. Med Sci Sports Exerc 49:2469–2477. https://doi.org/10.1249/MSS.0000000000001390
Nightingale TE, Metcalfe RS, Vollaard NB, Bilzon JL (2017b) Exercise guidelines to promote cardiometabolic health in spinal cord injured humans: time to raise the intensity? Arch Phys Med Rehabil 98:1693–1704. https://doi.org/10.1016/j.apmr.2016.12.008
Nyberg M, Mortensen SP, Hellsten Y (2013) Physical activity opposes the age-related increase in skeletal muscle and plasma endothelin-1 levels and normalizes plasma endothelin-1 levels in individuals with essential hypertension. Acta Physiol 207:524–535. https://doi.org/10.1111/apha.12048
Nyberg M, Seidelin K, Andersen TR, Overby NN, Hellsten Y, Bangsbo J (2014) Biomarkers of vascular function in premenopausal and recent postmenopausal women of similar age: effect of exercise training. Am J Physiol Regul Integr Comp Physiol 306:510–517. https://doi.org/10.1152/ajpregu.00539.2013
Pelletier CA, Totosy De Zepetnek JO, Macdonald MJ, Hicks AL (2015) A 16-week randomized controlled trial evaluating the physical activity guidelines for adults with spinal cord injury. Spinal Cord 53:363–367. https://doi.org/10.1038/sc.2014.167
Pyke KE, Tschakovsky ME (2007) Peak vs. total reactive hyperemia: Which determines the magnitude of flow-mediated dilation? J Appl Physiol 102:1510–1519. https://doi.org/10.1152/japplphysiol.01024.2006
Ravensbergen HJC, Lear SA, Claydon VE (2014) Waist circumference is the best index for obesity-related cardiovascular disease risk in individuals with spinal cord injury. J Neurotrauma 31:292–300. https://doi.org/10.1089/neu.2013.3042
Riebe D, Ehrman JK, Liguori G, Magal M (2018) ACSM’s guidelines for exercise testing and prescription. Wolters Kluwer, Philadelphia
Rosety-Rodriguez M, Camacho A, Rosety I, Fornieles G, Rosety MA, Diaz AJ et al (2014) Low-grade systemic inflammation and leptin levels were improved by arm cranking exercise in adults with chronic spinal cord injury. Arch Phys Med Rehabil 95:297–302. https://doi.org/10.1016/j.apmr.2013.08.246
Rowley NJ, Dawson EA, Hopman MTE, George KP, Whyte GP, Thijssen DHJ et al (2012) Conduit diameter and wall remodeling in elite athletes and spinal cord injury. Med Sci Sports Exerc 44:844–849. https://doi.org/10.1249/MSS.0b013e31823f6887
Sawatzky B, Herrington B, Choi K, Ben Mortenson W, Borisoff J, Sparrey C et al (2022) Acute physiological comparison of sub-maximal exercise on a novel adapted rowing machine and arm crank ergometry in people with a spinal cord injury. Spinal Cord. https://doi.org/10.1038/s41393-022-00757-2
Stoner L, Sabatier M, VanhHiel L, Groves D, Ripley D, Palardy G et al (2006) Upper vs lower extremity arterial function after spinal cord injury. J Spinal Cord Med 29:138–146. https://doi.org/10.1080/10790268.2006.11753867
ter Avest E, Holewijn S, Stalenhoef A, de Graaf J (2005) Variation in non-invasive measurements of vascular function in healthy volunteers during daytime. Clin Sci 108:425–431
Thijssen DHJ, Rongen GA, Smits P, Hopman MTE (2008) Physical (in) activity and endothelium-derived constricting factors: overlooked adaptations. J Physiol 2:319–324. https://doi.org/10.1113/jphysiol.2007.145698
Thijssen DHJ, Bruno RM, Van Mil ACCM, Holder SM, Faita F, Greyling A et al (2019) Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J 40:2534–2547. https://doi.org/10.1093/eurheartj/ehz350
Thompson PD, Buchner D, Piña IL, Balady GJ, Williams MA, Marcus BH et al (2003) Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the council on clinical cardiology (subcommittee on exercise, rehabilitation, and prevention) and the council on nutrition, physical. Circulation 107:3109–3116. https://doi.org/10.1161/01.CIR.0000075572.40158.77
Tinken TM, Thijssen DHJ, Black MA, Cable NT, Green DJ (2008) Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol 586:5003–5012. https://doi.org/10.1113/jphysiol.2008.158014
Totosy de Zepetnek JO, Pelletier CA, Hicks AL, MacDonald MJ (2015) Following the physical activity guidelines for adults with spinal cord injury for 16 weeks does not improve vascular health: a randomized controlled trial. Arch Phys Med Rehabil 96:1566–1575. https://doi.org/10.1016/j.apmr.2015.05.019
Troy KL (2011) Biomechanical validation of upper extremity exercise in wheelchair users: design considerations and improvements in a prototype device. Disabil Rehabil Assist Technol 6:22–28. https://doi.org/10.3109/17483107.2010.509883
Tweedy SM, Beckman EM, Geraghty TJ, Theisen D, Perret C, Harvey LA et al (2017) Exercise and sports science Australia (ESSA) position statement on exercise and spinal cord injury. J Sci Med Sport 20:108–115. https://doi.org/10.1016/j.jsams.2016.02.001
Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97:1837–1847. https://doi.org/10.1161/01.CIR.97.18.1837
Acknowledgements
We thank the participants for their commitment to the study, J. L.J. de Wit for assistance with the exercise training sessions and testing, R.W. Rasmussen and A. L. Larsen for conducting blood sample analyses. We would also like to acknowledge suppliers of training equipment (Aalborg Rowing Club for lending of a Concept 2 ergometer; Modest Sport for sponsoring Adapt2row units; and iQniter for sponsoring heart rate belts), energy bars to participants (Med24.dk), and Wolturnus A/S for lending equipment for the laboratory test.
Funding
This work is partially supported by The Aage and Johanne Louis-Hansens Foundation (20-2B-5947; RKH).
Author information
Authors and Affiliations
Contributions
RKH, AS, UL, AH, DHJT, LG, RGL conceived and designed research. Data collection and analysis were performed by RKH, AH, MM, KF. The first draft of the manuscript was written by RKH and RGL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Ethical approval
Approval was obtained from the Ethics Committee of North Denmark (N-20190053) and conducted in accordance with the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Communicated by Massimo Pagani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hansen, R.K., Samani, A., Laessoe, U. et al. Rowing exercise increases cardiorespiratory fitness and brachial artery diameter but not traditional cardiometabolic risk factors in spinal cord-injured humans. Eur J Appl Physiol 123, 1241–1255 (2023). https://doi.org/10.1007/s00421-023-05146-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05146-y