Abstract
For centuries, cold temperatures have been used by humans for therapeutic, health and sporting recovery purposes. This application of cold for therapeutic purposes is regularly referred to as cryotherapy. Cryotherapies including ice, cold-water and cold air have been popularised by an ability to remove heat, reduce core and tissue temperatures, and alter blood flow in humans. The resulting downstream effects upon human physiologies providing benefits that include a reduced perception of pain, or analgesia, and an improved sensation of well-being. Ultimately, such benefits have been translated into therapies that may assist in improving post-exercise recovery, with further investigations assessing the role that cryotherapies can play in attenuating the ensuing post-exercise inflammatory response. Whilst considerable progress has been made in our understanding of the mechanistic changes associated with adopting cryotherapies, research focus tends to look towards the future rather than to the past. It has been suggested that this might be due to the notion of progress being defined as change over time from lower to higher states of knowledge. However, a historical perspective, studying a subject in light of its earliest phase and subsequent evolution, could help sharpen one’s vision of the present; helping to generate new research questions as well as look at old questions in new ways. Therefore, the aim of this brief historical perspective is to highlight the origins of the many arms of this popular recovery and treatment technique, whilst further assessing the changing face of cryotherapy. We conclude by discussing what lies ahead in the future for cold-application techniques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
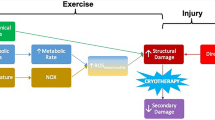
Cryotherapy is an umbrella term often used to describe therapeutic processes involving cold temperatures (Fig. 1). The principle purpose of cryotherapy is the withdrawal of heat (Kwiecien and McHugh 2021) assisted by way of reductions in core and tissue temperatures (Stephens et al. 2018; Vromans et al. 2019) and alterations in blood flow (Mawhinney et al. 2017a, b, 2020). Physiologically, the efficacy of cryotherapy is seen to be important primarily due to analgesic benefits (Murray and Cardinale 2015) associated with slowing sensory nerve conduction velocity (Herrera et al. 2010; Algafly and George 2007; Ernst and Fialka 1994). Historically, this has led to cryotherapy gaining much attention (Fig. 2), with its application long been thought as efficient in the treatment of primary and secondary tissue damage and the ensuing inflammatory response. However, in-depth discussion of the underpinning physiology of cryotherapy is beyond the scope of the current article; as such, readers are directed to recent reviews highlighted by ourselves and others (Kwiecien and McHugh 2021; Peake et al. 2017).
Interest in cryotherapy research since 1950. Data were collected using the “europepmc” R package (Jahn and Salmon 2021) using the search term “cryotherapy” and displayed as a percentage of all publications in the given year. The year-to-year trend is displayed by overlaying a loess smoothed fit curve on the data points
Over time, the way in which cold temperature has been applied to the human body has remained largely unchanged, with the application of ice, cold-air and cold-water maintaining popularity. The evolution of practice seemingly focussed on the cold dose applied through strict control of the temperature and duration of the cooling stimulus. Many aspects of historical physiology can be traced as far back as ancient Greece (West 2014). Indeed, the use of cryotherapy for benefits to health, treatment and recovery dates back centuries. Work by Hippocrates suggested water therapy could ‘allay lassitude’ in reducing the depletion of energy or strength (Tipton et al. 2017); the mention of ice and snow in relation to oedema leading to some crediting him as the grandfather of cryotherapy (Kwiecien and McHugh 2021; Rivenburgh 1992). Whilst considerable progress has been made in our understanding of the mechanistic changes associated with adopting cryotherapies, research focus tends to look towards the future rather than to the past. Reviewing a topic with a historical perspective, studying a subject considering its earliest phase and subsequent evolution, may help sharpen one’s vision of the present; helping to generate new research questions as well as look at old questions in new ways (Lawrence 1984). Therefore, the aim of this brief historical perspective is to highlight the origins of the many arms of this popular recovery and treatment technique, whilst further assessing the changing face of cryotherapy and discussing what lies ahead in the future for cold-application techniques.
History of cold-water immersion
The beneficial effects of cold-water immersion (CWI) on human physiology dates as far back as 3500BC with the Edwin Smith Papyrus making numerous references to cold being used for therapeutic purposes (Wang et al. 2006). Ancient Greeks utilised cold-water for therapies as well as relaxation and socialisation; Hippocrates in fourth century BC documenting the use of cold for medicinal purposes and analgesic benefits (Tsoucalas et al. 2015). In Hippocrates’ work entitled “On airs, waters and places”, he makes the statement that “the water can cure everything,” further emphasising its value (Hippocrates, translated by Lloyd, Chadwick & Mann 1950). Cold-water immersion was traditionally used as a treatment for fever with the Roman physician Claudius Galen advocating its use for tertian fever (Wang et al. 2006). The same notion appears hundreds of years later in the work of the physiologist James Currie, who utilised cold-water for treatment of his own fevers (Forrester 2000; Cosby 1950). Currie furthered his interests in the impact of cold-water on human physiology by investigating its influence upon body temperature, pulse, and respiration amongst other parameters. He documented the first records of human temperatures in health, disease, and experimental conditions (Henderson 1971); carrying out such experiments in his own “water cure establishment” to prove the value of hydrotherapy (Tsoucalas et al. 2015).
In the early twentieth century, the physician Edgar A. Hines Jr (1906–1978) advanced our understanding of the physiological responses to cold-water immersion by building upon Bayard T. Horton’s 1927 earlier work describing cold allergy (Lamotte et al. 2021). Hines’ contribution to the scientific and clinical community was to develop the cold-pressor test to study blood pressure variability. In his 1932 seminal paper (Hines and Brown 1932), he showed that immersing the hands in cold-water (4–5 °C for 30 s) led to a different magnitude and time-course of blood pressure response in subjects presenting with hypertension. Hines’ follow-up investigations (Hines and Brown 1933) examining vasomotor reactions to selective sympathectomy being fundamental in interpreting autonomic control of the cold pressor response. Indeed, we now understand that concomitant stimulation of the sympathetic (cold shock) and parasympathetic (diving response; Gooden 1994) arms of the nervous system can lead to autonomic conflict and cardiac arrhythmias (Tipton et al. 2010).
It was not until the 1960s and the work by D H Clarke that cold-water immersion was investigated for benefits to post-exercise recovery (Clarke 1963; Clarke and Stelmach 1966). However, in the decades that followed, attention quickly focussed back towards survival during cold-water exposure; Prof. Mike Tipton (MBE) conducting much work in examining cold shock responses (Tipton 1989), impact of different clothing ensembles (Tipton and Golden 1987; Tipton and Vincent 1989) and adaptations to multiple water immersions (Golden and Tipton 1988). It was at the latter end of the 1990s that the focus of work returned towards determining cold-water immersion’s effects upon performance recovery. Many researchers have since followed the path taken by Paddon-Jones and Quigley (1997) by adopting exercise-induced muscle damaging (eccentric) protocols to track functional, inflammatory and psychophysical responses over several days after cooling. At the turn of the new millennium, a plethora of similar studies followed, employing different exercise modalities, subject cohorts, and/or cooling doses (i.e., duration, water temperature, water depth); the primary aim to inform sports practice. The sheer volume of published studies has led to meta-analyses (Leeder et al. 2012; Poppendieck et al. 2013; Hohenauer et al. 2015; Machado et al. 2016) becoming prevalent in the empirical literature, thus helping form a consensus around the application of cold-water immersion. Current recommendations for the use of CWI derived from these meta-analyses suggest a protocol of 10–15 min at 10–15 °C (Machado et al. 2016) to promote effective recovery, whilst a dose of 1.1 (i.e., 11 min at 10 °C) is required to significantly reduce muscle tissue temperature (Vromans et al. 2019). Elsewhere, it has been established that immediate immersion is preferable versus delayed immersion (Brophy-Williams et al. 2011), whilst the depth of immersion is unlikely to play a significant role (Leeder et al. 2015).
Nevertheless, despite the significant interest, the underpinning physiological mechanisms at the level of the muscle remained largely overlooked. Over the last decade, work shifted towards understanding the central roles that post-immersion changes in muscle temperature (reduction up to − 6.4 °C; Freitage et al. 2021), and limb and cutaneous blood flow (20–30% reduction vascular conductance; Gregson et al. 2011) play in influencing the recovery process. Later studies made use of technological advances to progress earlier work in this area assessing cooling-induced changes in muscle blood flow per se (Ihsan et al. 2013; Mawhinney et al. 2020; Choo et al. 2018). Recent advances in the field of cellular and molecular physiology have also enabled regulatory mechanisms in human skeletal muscle to be studied, developing our insight into important pathways involved in endurance (Joo et al. 2016; Ihsan et al. 2015) and strength (Roberts et al. 2015; Fyfe et al. 2019; Peake et al. 2020) adaptation after cold-water exposure.
History of ice application
Ice is perhaps recognised as the most traditional mode of cryotherapy; Napoleon’s surgeon to the Grand Army, Baron Dominique Larrey, being one of the earliest proponents in recommending ice and snow to assist in painless amputations and operations on soldiers (Henderson 1971). In the late 1840s, this concept was extended by the physician James Arnott who started using local application of salt solution containing crushed ice to freeze cancerous tumours (Theodorescu 2004); thus, unwittingly developing cryosurgery. However, it was not until the 1960s that the application of ice was recommended for the therapeutic treatment of musculoskeletal injury (Grant 1964; Hayden 1964).
The ability to decrease tissue temperature is fundamental to ice’s therapeutic benefit. In 1955, Bierman studied how ice pack application (120 min) could markedly reduce (~ 6 °C) skin surface temperature (Bierman 1955). Similar studies followed, employing various methods (spray, refrigerant gel, ice pack, ice massage) and cooling durations to subsequently report skin temperature reductions between 6 and 30 °C (see review by Meeusen and Lievens 1986). Indeed, Bleakley and Hopkins (2010) highlighted that crushed ice can reduce skin temperature to below 10 °C after anywhere between 5 and 20 min application. At around the same period as Bierman’s work, Bing and colleagues were one of the first to document the intramuscular temperature change (at 3 cm depth) to ice pack application (Bing et al. 1945). Waylonis (1967) subsequently providing a more comprehensive assessment by recording incremental (0.5 cm) muscle temperature changes to ice massage. However, it should be noted that subcutaneous adipose tissue will influence such temperatures, with Bleakley and Hopkins (2010) highlighting the lowest temperatures seen often coincide with smaller thigh skinfold thickness. It has been suggested that reductions in muscle temperature are related to slowing metabolism, lessening the need for cellular oxygen in the acute period after soft-tissue injury (Swenson et al. 1996). In this regard, much of our current knowledge can be derived back to investigations to conserve organ tissue for transplantation and limb replantation (Sapega et al. 1988; Krezdorn et al. 2017). An early pioneer being the surgeon R. Y. Calne in 1963, who demonstrated that kidneys extracted from Mongrel dogs and cooled with chipped ice could extend the period of ischemia (Calne et al. 1963). Calne’s histochemical analyses showing decreased cellular necrosis within the organ; what we would now term a decrease in secondary ischemic injury (Merrick et al. 1999).
In the 1970s, investigations commenced to better understand the analgesic effect of ice upon the pain threshold (Halliday et al. 1969; Benson and Copp 1974; Bugaj 1975). Bugaj (1975), demonstrating ice massage to acutely abolish the pain threshold (to surface pin prick) when skin temperature was reduced to 13.6 °C. It is now understood that a large part of the analgesic benefit experienced is because of a reduced nerve conduction velocity in sensory nerves (Herrera et al. 2010; Algafly and George 2007; Ernst and Fialka 1994). In 1978, Gabe Mirkin was no doubt influenced by past findings to introduce the widely circulated RICE acronym (rest, ice, compression, and elevation) to guide acute sport injury management. The original acronym subsequently adapted to include RICES (rest, ice, compression, elevation, and stabilisation) (Long and Jutte 2020) and PRICE (protection, rest, ice, compression, and elevation) (Bleakley et al. 2007).
Despite the longstanding popularity of applying ice in sport injury management, the evidence for its use in humans is limited; however, Guillot and colleagues were able to show a positive impact of ice application upon inflammatory cytokines (Guillot et al. 2017, 2019). Nevertheless, recent literature has questioned the use of traditional cold therapy in the early phases of soft-tissue injury (Wang and Ni 2021) or suggests the removal of ice altogether in the management of soft-tissue injuries (Dubois and Esculier 2020). Consequently, the acronyms of PEACE (protection, elevation, avoid anti-inflammatory drugs, compression, and education) and LOVE (load, optimism, vascularization, and exercise) have emerged (Dubois and Esculier 2020). Whilst ice application for acute injury management is still often witnessed pitch side for sporting contact and non-contact injuries, it has more recently been transferred to form part of a periodized recovery approach to facilitate performance (Thorpe 2021).
History of cold air application
Whilst the use of cold-water and ice cryotherapy methods for exercise recovery have been long established, the application of extreme cold air temperatures (below − 100 °C) is a relatively new technique in the sporting world. The typical use of cold air for recovery is in the form of whole-body cryotherapy (WBC) chambers, which typically exposes an individual to 2–3 min of exposure, after a preceding 30 s temperature adaptation period at approximately 60 °C (Banfi et al. 2010). The cold air, typically administered in the form of either liquid nitrogen or refrigerated cold air (Costello et al. 2016), is proposed to be effective in reducing the sensation of delayed onset of muscle soreness (Hausswirth et al. 2011), increasing parasympathetic activation (Hausswirth et al. 2013) and anti-inflammatory cytokines (Lubkowska et al. 2011; Lombardi et al. 2017).
Initial WBC chambers were built in Japan around 1978, through pioneering work led by Dr. Toshima Yamaguchi for the treatment of rheumatoid arthritis and general pain management. Dr. Yamaguchi’s initial work found that exposure to WBC led to rapid temperature decreases on the outer layers of the person’s skin, resulting in a release of endorphins and subsequent reduction in subjective pain assessment. Indeed, it was reported that around 80% of Dr. Yamaguchi’s patients were completely relieved of their symptoms and chronic pain issues. The success of WBC treatment in Japan led to data being presented at the Rheumatology Congress in 1979; promoting the expansion of its use worldwide. The renowned German rheumatologist, Prof. Reinhard Fricke, bringing WBC treatment to Europe in 1984 and setting up a cryotherapeutic module for hundreds of patients suffering from multiple sclerosis and arthritic conditions.
Despite the popularisation of WBC for the treatment of various conditions, it was not until the past decade that its use crossed over into the sporting world for exercise recovery. Sports such as rugby and American football were early adopters of WBC chambers for recovery, mainly due to the proposed reduction in inflammation that is common in contact sports (Selfe et al. 2014). It is suggested that the extreme temperatures of WBC magnify the effect of cold therapies (such as in comparison to CWI), thus potentially shortening recovery time (Rose et al. 2017). Anecdotally, athletes have reported preferring the use of ‘dry’ therapies such as WBC rather than prolonged immersion in CWI; one reason for its increased popularisation. In the early 2010s, commercial companies were providing sporting organisations with permanent WBC chambers installed within their training base. However, the lack of portability of such chambers limited their practical application. This has led to more recent developments, such as transportable WBC chambers, that can be mounted on a truck for a semi-permanent installation.
Alongside the developments of WBC, a new method called partial-body cryotherapy (PBC) using a portable cryotherapy-cabin has gained popularity within the general public. This system has an open tank and exposes the body, except the head and neck, to similar extreme cold air temperatures (Costello et al. 2016). Whilst PBC has been widely adopted due to its proposed benefits for beauty treatment (e.g., skin care improvements), recreational athletes have started to use such methods for recovery after exercise. PBC cabins have become more widely available in health centres at an affordable cost. Unfortunately, this has coincided with reported cases of severe injury (e.g., skin burns) and death as a result cold air therapy misuse. Moreover, as adverse events must be self-reported, it is likely that the number of events has not been comprehensively documented. Currently, the efficacy of PBC cabins compared to WBC for exercise recovery has yet to be fully determined, with the long-term use of cold air therapy methods (for recovery) still remaining to be clearly established (Malone et al. 2021).
The changing face of cryotherapy
As the popularity of cryotherapy has increased, clinicians, practitioners, and athletes alike have pursued easy to use, rapid to deploy, and portable cryotherapy alternatives. As an example, development of cryopneumatic devices (e.g., Polar Care, Breg, Inc.; Game Ready, CoolSystems, Inc.; etc.) became popular after receiving patents in the 1990s and early 2000s. Initially implemented for recovery following surgical procedures, such as knee arthroplasty (Su et al. 2012; Murgier et al. 2017; Schinsky et al. 2016), knee arthroscopy (Waterman et al. 2012; Murgier and Cassard 2014), hip arthroplasty (Leegwater et al. 2012), hip arthroscopy (Klaber et al. 2019), and spine surgery (Nabıyev et al. 2018; Bellon et al. 2019), these devices deliver continuous or intermittent compression whilst simultaneously supplying low temperature “exchange fluid” to the injured area. Importantly, they can be applied for as long a time period as desired without substantial change in the temperature of the material contacting the affected area. The result from these studies is highlighting the acute analgesic effect and consistent reduction in patient reported pain. These apparatus presented the advantage of portability and thus were extremely well received by athletes for recovery from exercise. However, to date, only one study has investigated the effect of a cryopneumatic device (as a recovery method) upon physiological and biomechanical outcomes following exercise; showing no benefit in accelerating acute recovery of strength loss (hamstring eccentric strength; Alexander et al. 2021). Ultimately, evidence for the use of cryopneumatic devices for exercise recovery remain anecdotal.
More recently, phase change material (PCM) has been introduced as a modern cryotherapy modality for recovery following exercise (see Kwiecien et al. 2020a for review). A PCM is any substance that goes through a phase transition between states of matter with no detectible temperature change (defined as a latent phase). On the contrary, a substance like water or a gel pack (which does not undergo a change in phase) only experiences a temperature change that can be felt and measured by a thermometer (defined as a sensible phase). A modality with PCM properties is advantageous, because it can absorb large amounts of heat at an almost constant temperature until all the materials are melted; resulting in lower intramuscular temperatures than those which maintain the same phase (Chesterton et al. 2002; Merrick et al. 2003; Vieira et al. 2013). Compared to the conventional cryotherapy interventions, PCM offers an advantage over traditional cryotherapy modalities in that it can be safely administered for prolonged durations (Kwiecien et al. 2019), resulting in a larger magnitude of change in tissue temperature (Merrick et al. 2003; Dykstra et al. 2009).
In particular, PCM packs with a latent phase of 15 °C (Glacier Tek USDA BioPreferred PureTemp PCM, Plymouth, MN, USA) can be safely administered for durations of 3 (Brownstein et al. 2019; Clifford et al. 2018; Kwiecien et al. 2019, 2021; Mullaney et al. 2021) to 6 (Kwiecien et al. 2018; Kwiecien et al. 2020b) h at a time in one dose. Indeed, 6 h of 15 °C PCM cooling has been shown to accelerate recovery of strength loss and soreness following isolated eccentric exercise of the quadriceps muscle in untrained (Kwiecien et al. 2018) and trained (Kwiecien et al. 2020b) individuals. Similarly, 3 h of PCM cooling has also been shown to accelerate recovery of strength loss (Brownstein et al. 2019; Clifford et al. 2018) and soreness (Clifford et al. 2018) following soccer match play and baseball pitching (Mullaney et al. 2021) but not following a marathon run (Kwiecien et al. 2021). Nevertheless, the efficacy of prolonged PCM cooling for accelerating recovery from “injury” remains to be investigated. It should be noted that anecdotal application of these evolving techniques likely includes multiple and combined use. However, as research surrounding these techniques is emerging, the interchangeability of these cryotherapy alternatives is currently unknown.
The future of cryotherapy
Whilst the method of application is open to change, the mechanisms of action remain the same. Whether the cooling stimulus applied is water, ice, air, or PCM, future research should direct attention towards ensuring that appropriate protocols are utilised. In this sense, an appropriate protocol should be able to elicit the necessary physiological alterations proposed to benefit health, injury, or recovery (Kwiecien and McHugh 2021). This is undoubtedly likely to change depending on the method of cryotherapy used. Indeed, an important point to note is the impact of the thermal gradient created between the skin and the surrounding environment (Bleakley et al. 2014). The thermal conductivity, or heat-transfer co-efficient, is much greater for ice (2.18 k), when compared with water (0.58 k) and air (0.024 k), suggesting a greater ability for the removal of heat from the body using ice. However, despite these values, water and air may be more efficient through greater surface area contact (Bleakley et al. 2014). Therefore, the thermal properties and rate of heat exchange, temperature and duration of cooling, and size of area exposed to cooling should all be carefully considered. With data existing directly comparing the efficacy of cryotherapy modalities on physiological parameters (Abaïdia et al. 2017; Costello et al. 2012; Wilson et al. 2018; Mawhinney et al. 2017b), it is important that practitioners are aware that some methods may be more efficient in the removal of heat than others.
One thing that remains important is the correct and efficient transfer of knowledge between scientific and applied communities (Allan et al. 2021). When applying scientific data to practice, it is important that the correct context is utilised (Ihsan et al. 2021). Recent expert views suggest cryotherapies that aim to benefit or improve health, injury, or recovery should be implemented in an individualised and periodised manner that takes into account the athlete, training and competition schedule, session aims, proximity of future exercise, and environmental conditions (Ihsan et al. 2021; Ihsan et al. 2020; Allan and Mawhinney 2017, Grainger et al. 2021). Whilst readers are directed to recent useful reviews highlighting the positive effects of cryotherapies upon health, injury, and recovery (Kwiecien and McHugh 2021), they should also be aware of important caveats that might arise in specific situations; for example, the paradox whereby post-exercise cooling might enhance endurance-based adaptations in skeletal muscle (Ihsan et al. 2014, 2015, 2020; Aguiar et al. 2016; Joo et al. 2016; Allan et al. 2017, 2019, 2020; Broatch et al. 2017) but dampen hypertrophic aims (Roberts et al. 2015; Fuchs et al. 2020). A point we have previously discussed (Ihsan et al. 2021) and one that serves to emphasise the need for individualisation and periodisation of recovery strategies.
What does remain clear is the firm centuries old belief in cryotherapy techniques and their analgesic properties. Recent data, focussing on the perception of one post-exercise cooling strategy (CWI), highlight the positive perception surrounding its application, with “end-user belief” being an important variable considered before use (Allan et al. 2021). Whilst we now have the capabilities to examine these age-old theories, researchers should look to further establish the importance of cooling on health, wellness, and well-being.
Availability of data and material
Not applicable.
Abbreviations
- CWI:
-
Cold-water immersion
- PBC:
-
Partial-body cryotherapy
- PCM:
-
Phase change material
- WBC:
-
Whole-body cryotherapy
References
Abaïdia AE, Lamblin J, Delecroix B, Leduc C, McCall A, Nédélec M, Dawson B, Baquet G, Dupont G (2017) Recovery from exercise-induced muscle damage: cold-water immersion versus whole-body cryotherapy. Int J Sports Physiol Perform 12(3):402–409. https://doi.org/10.1123/ijspp.2016-0186
Aguiar PF et al (2016) Post-exercise cold water immersion does not alter high intensity interval training-induced exercise performance and Hsp72 responses, but enhances mitochondrial markers. Cell Stress Chaperones 21(5):793–804
Alexander J et al (2021) Recovery profiles of eccentric hamstring strength in response to cooling and compression. J Bodyw Mov Ther 27:9–15
Algafly AA, George KP (2007) The effect of cryotherapy on nerve conduction velocity, pain threshold and pain tolerance. Br J Sports Med 41(6):365–369 (discussion 369)
Allan R, Mawhinney C (2017) Is the ice bath finally melting? Cold water immersion is no greater than active recovery upon local and systemic inflammatory cellular stress in humans. J Physiol 595(6):1857–1858
Allan R et al (2017) Postexercise cold water immersion modulates skeletal muscle PGC-1alpha mRNA expression in immersed and nonimmersed limbs: evidence of systemic regulation. J Appl Physiol (1985) 123(2):451–459
Allan R et al (2019) Low pre-exercise muscle glycogen availability offsets the effect of post-exercise cold water immersion in augmenting PGC-1alpha gene expression. Physiol Rep 7(11):e14082
Allan R et al (2020) PGC-1alpha alternative promoter (Exon 1b) controls augmentation of total PGC-1alpha gene expression in response to cold water immersion and low glycogen availability. Eur J Appl Physiol 120(11):2487–2493
Allan R et al (2021) Athlete, coach and practitioner knowledge and perceptions of post-exercise cold-water immersion for recovery: a qualitative and quantitative exploration. Sport Sci Health. https://doi.org/10.1007/s11332-021-00839-3
Banfi G et al (2010) Whole-body cryotherapy in athletes. Sports Med 40(6):509–517
Bellon M et al (2019) Efficacy of the Game Ready((R)) cooling device on postoperative analgesia after scoliosis surgery in children. Eur Spine J 28(6):1257–1264
Benson TB, Copp EP (1974) The effects of therapeutic forms of heat and ice on the pain threshold of the normal shoulder. Rheumatol Rehabil 13(2):101–104
Bierman W (1955) Therapeutic use of cold. J Am Med Assoc 157(14):1189–1192
Bing HI et al (1945) The effect on muscular temperature produced by cooling normal and ultraviolet radiated skin. Acta Med Scand 121:577–591
Bleakley CM, Hopkins JT (2010) Is it possible to achieve optimal levels of tissue cooling in cryotherapy? Phys Therapy Rev 15(4):344-350. https://www.tandfonline.com/action/showCitFormats?doi=10.1179/174328810X12786297204873
Bleakley CM et al (2007) The PRICE study (Protection Rest Ice Compression Elevation): design of a randomised controlled trial comparing standard versus cryokinetic ice applications in the management of acute ankle sprain [ISRCTN13903946]. BMC Musculoskelet Disord 8:125
Bleakley C, Bieuzen F, Davison G, Costello J (2014) Whole-body cryotherapy: empirical evidence and theoretical perspectives. Open Access J Sports Med 5:25–36. https://doi.org/10.2147/OAJSM.S41655
Broatch JR et al (2017) Cold-water immersion following sprint interval training does not alter endurance signaling pathways or training adaptations in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 313(4):R372–R384
Brophy-Williams N, Landers G, Wallman K (2011) Effect of immediate and delayed cold water immersion after a high intensity exercise session on subsequent run performance. J Sports Sci Med 10(4):665–670
Brownstein CG et al (2019) The effect of phase change material on recovery of neuromuscular function following competitive soccer match-play. Front Physiol 10:647
Bugaj R (1975) The cooling, analgesic, and rewarming effects of ice massage on localized skin. Phys Ther 55(1):11–19
Calne RY et al (1963) Renal preservation by ice-cooling: an experimental study relating to kidney transplantation from cadavers. Br Med J 2(5358):651–655
Chesterton LS et al (2002) Skin temperature response to cryotherapy. Arch Phys Med Rehabil 83(4):543–549
Choo HC et al (2018) Peripheral blood flow changes in response to postexercise cold water immersion. Clin Physiol Funct Imaging 38(1):46–55
Clarke DH (1963) Effect of immersion in hot and cold water upon recovery of muscular strength following fatiguing isometric exercise. Arch Phys Med Rehabil 44:565–568
Clarke DH, Stelmach GE (1966) Muscular fatigue and recovery curve parameters at various temperatures. Res Q 37(4):468–479
Clifford T et al (2018) Cryotherapy reinvented: application of phase change material for recovery in elite soccer. Int J Sports Physiol Perform 13(5):584–589
Cosby CB (1950) James Currie and hydrotherapy. J Hist Med Allied Sci 5(3):280–288
Costello JT, Culligan K, Selfe J, Donnelly AE (2012) Muscle, skin and core temperature after -110°c cold air and 8°c water treatment. PLoS ONE 7(11):e48190. https://doi.org/10.1371/journal.pone.0048190
Costello JT et al (2016) Cochrane review: Whole-body cryotherapy (extreme cold air exposure) for preventing and treating muscle soreness after exercise in adults. J Evid Based Med 9(1):43–44
Dubois B, Esculier JF (2020) Soft-tissue injuries simply need PEACE and LOVE. Br J Sports Med 54(2):72–73
Dykstra JH et al (2009) Comparisons of cubed ice, crushed ice, and wetted ice on intramuscular and surface temperature changes. J Athl Train 44(2):136–141
Ernst E, Fialka V (1994) Ice freezes pain? A review of the clinical effectiveness of analgesic cold therapy. J Pain Symptom Manage 9(1):56–59
Forrester JM (2000) The origins and fate of James Currie’s cold water treatment for fever. Med Hist 44(1):57–74
Freitag L, Clijsen R, Deflorin C, Taube W, Taeymans J, Hohenauer E (2021) Intramuscular temperature changes in the quadriceps femoris muscle after post-exercise cold-water immersion (10°c for 10 min): a systematic review with meta-analysis. Front Sports Act living 3:660092. https://doi.org/10.3389/fspor.2021.660092
Fuchs CJ et al (2020) Postexercise cooling impairs muscle protein synthesis rates in recreational athletes. J Physiol 598(4):755–772
Fyfe JJ et al (2019) Cold water immersion attenuates anabolic signaling and skeletal muscle fiber hypertrophy, but not strength gain, following whole-body resistance training. J Appl Physiol (1985) 127(5):1403–1418
Golden FS, Tipton MJ (1988) Human adaptation to repeated cold immersions. J Physiol 396:349–363
Gooden BA (1994) Mechanism of the human diving response. Integr Physiol Behav Sci 29(1):6–16
Grainger et al (2021) unpublished BASES expert statement. In review. https://www.bases.org.uk/imgs/9193_bas_bases_tses_winter_2021_online_expert_statement736.pdf
Grant AE (1964) Massage with ice (cryokinetics) in the treatment of painful conditions of the musculoskeletal system. Arch Phys Med Rehabil 45:233–238
Gregson W et al (2011) Influence of cold water immersion on limb and cutaneous blood flow at rest. Am J Sports Med 39(6):1316–1323
Guillot X, Martin H, Seguin-Py S, Maguin-Gaté K, Moretto J, Totoson P, Wendling D, Demougeot C, Tordi N (2017) Local cryotherapy improves adjuvant-induced arthritis through down-regulation of IL-6 / IL-17 pathway but independently of TNFα. PLoS ONE 12(7):e0178668. https://doi.org/10.1371/journal.pone.0178668
Guillot X, Tordi N, Laheurte C, Pazart L, Prati C, Saas P, Wendling D (2019) Local ice cryotherapy decreases synovial interleukin 6, interleukin 1β, vascular endothelial growth factor, prostaglandin-E2, and nuclear factor kappa B p65 in human knee arthritis: a controlled study. Arthritis Res Ther 21(1):180. https://doi.org/10.1186/s13075-019-1965-0
Halliday SM et al (1969) A trial of ice therapy and exercise in chronic arthritis. Physiotherapy 55(2):51–56
Hausswirth C et al (2011) Effects of whole-body cryotherapy vs. far-infrared vs. passive modalities on recovery from exercise-induced muscle damage in highly-trained runners. PLoS ONE 6(12):e27749
Hausswirth C et al (2013) Parasympathetic activity and blood catecholamine responses following a single partial-body cryostimulation and a whole-body cryostimulation. PLoS ONE 8(8):e72658
Hayden CA (1964) Cryokinetics in an early treatment program. Phys Ther 44:990–993
Henderson AR (1971) Cold–man’s assiduous remedy. Med Ann Dist Columbia 40(9):583–588
Herrera E et al (2010) Motor and sensory nerve conduction are affected differently by ice pack, ice massage, and cold water immersion. Phys Ther 90(4):581–591
Hines E, Brown GE (1932) A standard stimulus for measuring vasomotor reactions: Its application in the study of hypertension. Proc Staff Meet Mayo Clin 7:332–335
Hines EA, Brown GE (1933) A standard test for measuring the variability of blood pressure: its significance as an index of the prehypertensive state. Ann Intern Med 7:209–217
Hohenauer E et al (2015) The effect of post-exercise cryotherapy on recovery characteristics: a systematic review and meta-analysis. PLoS ONE 10(9):e0139028
Ihsan M et al (2013) Influence of postexercise cooling on muscle oxygenation and blood volume changes. Med Sci Sports Exerc 45(5):876–882
Ihsan M et al (2014) Postexercise muscle cooling enhances gene expression of PGC-1alpha. Med Sci Sports Exerc 46(10):1900–1907
Ihsan M et al (2015) Regular postexercise cooling enhances mitochondrial biogenesis through AMPK and p38 MAPK in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 309(3):R286-294
Ihsan M et al (2020) "Warming to the ice bath: Don't go cool on cold water immersion just yet!: Comment on: 1) Arthur J. Cheng. Cooling down the use of cryotherapy for post-exercise skeletal muscle recovery. Temperature. 2018; 5(2): 103–105. doi: https://doi.org/10.1080/23328940.2017.1413284. 2) Cheng et al. Post-exercise recovery of contractile function and endurance in humans and mice is accelerated by heating and slowed by cooling skeletal muscle. Journal of Physiology. 2017; 595(24): 7413–7426. doi: https://doi.org/10.1113/JP274870." Temperature (Austin) 7(3):223–225
Ihsan M et al (2021) Adaptations to post-exercise cold water immersion: friend, foe, or futile? Front Sports Act Living 3:714148
Jahn N, Salmon M (2021) “europepmc: R Interface to the Europe PubMed Central RESTful Web Service.” https://CRAN.R-project.org/package=europepmc
Joo CH et al (2016) Passive and post-exercise cold-water immersion augments PGC-1alpha and VEGF expression in human skeletal muscle. Eur J Appl Physiol 116(11–12):2315–2326
Klaber I et al (2019) Compressive cryotherapy is superior to cryotherapy alone in reducing pain after hip arthroscopy. J Hip Preserv Surg 6(4):364–369
Krezdorn N et al (2017) Tissue conservation for transplantation. Innov Surg Sci 2(4):171–187
Kwiecien SY, McHugh MP (2021) The cold truth: the role of cryotherapy in the treatment of injury and recovery from exercise. Eur J Appl Physiol 121(8):2125–2142
Kwiecien SY et al (2018) The efficacy of cooling with phase change material for the treatment of exercise-induced muscle damage: pilot study. J Sports Sci 36(4):407–413
Kwiecien SY et al (2019) Exploring the efficacy of a safe cryotherapy alternative: physiological temperature changes from cold-water immersion versus prolonged cooling of phase-change material. Int J Sports Physiol Perform 14:1288–1296
Kwiecien SY et al (2020a) Don’t lose your cool with cryotherapy: the application of phase change material for prolonged cooling in athletic recovery and beyond. Front Sports Act Living 2:118
Kwiecien SY et al (2020b) Prolonged cooling with phase change material enhances recovery and does not affect the subsequent repeated bout effect following exercise. Eur J Appl Physiol 120(2):413–423
Kwiecien SY et al (2021) Prolonging the duration of cooling does not enhance recovery following a marathon. Scand J Med Sci Sports 31(1):21–29
Lamotte G et al (2021) The expanding role of the cold pressor test: a brief history. Clin Auton Res 31(2):153–155
Lawrence B (1984) Historical perspective: Using the past to study the present. Acad Manage Rev 9(2):307–312
Leeder J et al (2012) Cold water immersion and recovery from strenuous exercise: a meta-analysis. Br J Sports Med 46(4):233–240
Leeder JD, van Someren KA, Bell PG et al (2015) Effects of seated and standing cold water immersion on recovery from repeated sprinting. J Sports Sci 33(15):1544–1552. https://doi.org/10.1080/02640414.2014.996914
Leegwater NC et al (2012) Cryocompression therapy after elective arthroplasty of the hip. Hip Int 22(5):527–533
Lombardi G et al (2017) Whole-body cryotherapy in athletes: from therapy to stimulation. An updated review of the literature. Front Physiol 8:258
Long BC, Jutte LS (2020) 21st century attacks on cryotherapy in sports health care—clinician beware. Athletic Training Sports Health Care 12(3):99–101
Lubkowska A et al (2011) The effect of prolonged whole-body cryostimulation treatment with different amounts of sessions on chosen pro- and anti-inflammatory cytokines levels in healthy men. Scand J Clin Lab Invest 71(5):419–425
Machado AF et al (2016) Can water temperature and immersion time influence the effect of cold water immersion on muscle soreness? A systematic review and meta-analysis. Sports Med 46(4):503–514
Malone J et al (2021) Effect of alterations in whole-body cryotherapy (WBC) exposure on post-match recovery markers in elite Premier League soccer players. Biol Sport 39:31–36
Mawhinney C et al (2017a) Influence of cold-water immersion on limb blood flow after resistance exercise. Eur J Sport Sci 17(5):519–529
Mawhinney C, Low DA, Jones H, Green DJ, Costello JT, Gregson W (2017b) Cold water mediates greater reductions in limb blood flow than whole body cryotherapy. Med Sci Sports Exerc 49(6):1252–1260. https://doi.org/10.1249/MSS.0000000000001223
Mawhinney C et al (2020) Changes in quadriceps femoris muscle perfusion following different degrees of cold-water immersion. J Appl Physiol (1985) 128(5):1392–1401
Meeusen R, Lievens P (1986) The use of cryotherapy in sports injuries. Sports Med 3(6):398–414
Merrick MA et al (1999) A preliminary examination of cryotherapy and secondary injury in skeletal muscle. Med Sci Sports Exerc 31(11):1516–1521
Merrick MA et al (2003) Cold modalities with different thermodynamic properties produce different surface and intramuscular temperatures. J Athl Train 38(1):28–33
Mullaney MJ et al (2021) Accelerated muscle recovery in baseball pitchers using phase change material cooling. Med Sci Sports Exerc 53(1):228–235
Murgier J, Cassard X (2014) Cryotherapy with dynamic intermittent compression for analgesia after anterior cruciate ligament reconstruction. Preliminary study. Orthop Traumatol Surg Res 100(3):309–312
Murgier J et al (2017) Cryotherapy with dynamic intermittent compression improves recovery from revision total knee arthroplasty. J Arthroplasty 32(9):2788–2791
Murray A, Cardinale M (2015) Cold applications for recovery in adolescent athletes: a systematic review and meta analysis. Extrem Physiol Med 4:17
Nabiyev VN et al (2018) Cryo-compression therapy after elective spinal surgery for pain management: a cross-sectional study with historical control. Neurospine 15(4):348–352
Paddon-Jones DJ, Quigley BM (1997) Effect of cryotherapy on muscle soreness and strength following eccentric exercise. Int J Sports Med 18(8):588–593
Peake JM et al (2017) Muscle damage and inflammation during recovery from exercise. J Appl Physiol (1985) 122(3):559–570
Peake JM et al (2020) The effects of cold water immersion and active recovery on molecular factors that regulate growth and remodeling of skeletal muscle after resistance exercise. Front Physiol. https://doi.org/10.3389/fphys.2020.00737
Poppendieck W et al (2013) Cooling and performance recovery of trained athletes: a meta-analytical review. Int J Sports Physiol Perform 8(3):227–242
Rivenburgh MSDW (1992) Physical modalities in the treatment of tendon injuries. Clin Sports Med 11(3):645–659
Roberts LA et al (2015) Post-exercise cold water immersion attenuates acute anabolic signalling and long-term adaptations in muscle to strength training. J Physiol 593(18):4285–4301
Rose C et al (2017) Whole-body cryotherapy as a recovery technique after exercise: a review of the literature. Int J Sports Med 38(14):1049–1060
Sapega AA et al (1988) The bioenergetics of preservation of limbs before replantation. The rationale for intermediate hypothermia. J Bone Joint Surg Am 70(10):1500–1513
Schinsky MF et al (2016) Multifaceted comparison of two cryotherapy devices used after total knee arthroplasty: cryotherapy device comparison. Orthop Nurs 35(5):309–316
Selfe J et al (2014) The effect of three different (-135 degrees C) whole body cryotherapy exposure durations on elite rugby league players. PLoS ONE 9(1):e86420
Stephens JM et al (2018) Core temperature responses to cold-water immersion recovery: a pooled-data analysis. Int J Sports Physiol Perform 13(7):917–925
Su EP et al (2012) A prospective, multi-center, randomised trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br 94(11 Suppl A):153–156
Swenson C et al (1996) Cryotherapy in sports medicine. Scand J Med Sci Sports 6(4):193–200
Theodorescu D (2004) Cancer cryotherapy: evolution and biology. Rev Urol 6(Suppl 4):S9–S19
Thorpe RT (2021) Post-exercise recovery: cooling and heating, a periodized approach. Front Sports Act Living 3:707503
Tipton MJ (1989) The initial responses to cold-water immersion in man. Clin Sci (lond) 77(6):581–588
Tipton MJ, Golden FS (1987) The influence of regional insulation on the initial responses to cold immersion. Aviat Space Environ Med 58(12):1192–1196
Tipton MJ, Vincent MJ (1989) Protection provided against the initial responses to cold immersion by a partial coverage wet suit. Aviat Space Environ Med 60(8):769–773
Tipton MJ et al (2010) ECG during helicopter underwater escape training. Aviat Space Environ Med 81(4):399–404
Tipton MJ et al (2017) Cold water immersion: kill or cure? Exp Physiol 102(11):1335–1355
Tsoucalas G et al (2015) Hydrotherapy: Historical landmarks of a cure all remedy. Archives Balkan Medical Union 50:430–432
Vieira A et al (2013) Cold modalities with different thermodynamic properties have similar effects on muscular performance and activation. Int J Sports Med 34(10):873–880
Vromans BA et al (2019) Cold water immersion settings for reducing muscle tissue temperature: a linear dose-response relationship. J Sports Med Phys Fitness 59(11):1861–1869
Wang ZR, Ni GX (2021) Is it time to put traditional cold therapy in rehabilitation of soft-tissue injuries out to pasture? World J Clin Cases 9(17):4116–4122
Wang H et al (2006) Cold as a therapeutic agent. Acta Neurochir (wien) 148(5):565–570 (discussion 569-570)
Waterman B et al (2012) The efficacy of combined cryotherapy and compression compared with cryotherapy alone following anterior cruciate ligament reconstruction. J Knee Surg 25(2):155–160
Waylonis GW (1967) The physiologic effects of ice massage. Arch Phys Med Rehabil 48(1):37–42
West JB (2014) Galen and the beginnings of Western physiology. Am J Physiol-Lung Cell Molecul Physiol 307(2):L121–L128
Wilson LJ, Cockburn E, Paice K, Sinclair S, Faki T, Hills FA, Gondek MB, Wood A, Dimitriou L (2018) Recovery following a marathon: a comparison of cold water immersion, whole body cryotherapy and a placebo control. Eur J Appl Physiol 118(1):153–163. https://doi.org/10.1007/s00421-017-3757-z
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
RA, WG, CM, MI, and SV conceived the idea. RA and CM edited introduction, cold-water, and future perspectives. JM edited cold air perspective. JA and CM edited ice application perspective. SK edited the changing face perspective. All authors contributed to final manuscript alterations.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Michael Lindinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Allan, R., Malone, J., Alexander, J. et al. Cold for centuries: a brief history of cryotherapies to improve health, injury and post-exercise recovery. Eur J Appl Physiol 122, 1153–1162 (2022). https://doi.org/10.1007/s00421-022-04915-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-022-04915-5