Abstract
Purpose
To investigate in vivo the adaptations of satellite cell induced by exercise performed in acute or chronic hypoxic conditions and their contribution to muscle remodeling and hypertrophy.
Methods
Search terms related to exercise, hypoxia and satellite cells were entered on Embase, PubMed and Scopus. Studies were selected for their relevance in terms of regulation of satellite cells by in vivo exercise and muscle contraction in hypoxic conditions.
Results
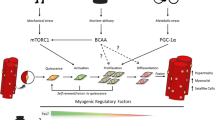
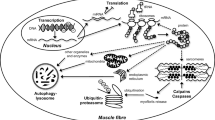
Satellite cell activation and proliferation seem to be enabled after acute hypoxic exercise via regulations induced by myogenic regulatory factors. Several studies reported also a role of the inflammatory pathway nuclear factor-kappa B and angiogenic factors such as vascular endothelial growth factor, both known to upregulate myogenesis. By stimulating angiogenesis, repeated exercise performed in acute hypoxia might contribute to satellite cell activation. Contrary to such exercise conditions, chronic exposure to hypoxia downregulates myogenesis despite the maintenance of physical activity. This impaired myogenesis might be induced by excessive oxidative stress and proteolysis.
Conclusion
In vivo studies suggest that, in comparison to exercise or hypoxia alone, exercise performed in a hypoxic environment, may improve or impair muscle remodeling induced by contractile activity depending upon the duration of hypoxia. Satellite cells seem to be major actors in these dichotomous adaptations. Further research on the role of angiogenesis, types of contraction and autophagy is needed for a better understanding of their respective role in hypoxic exercise-induced modulations of satellite cell activity in human.

Similar content being viewed by others
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Abbreviations
- CD:
-
Cluster of differentiation
- FGF:
-
Fibroblast growth factor
- HGF:
-
Hepatocyte growth factor
- HIF:
-
Hypoxia-inducible factor
- IGF:
-
Insulin-like growth factor
- IL:
-
Interleukin
- MHC:
-
Myosin heavy chain
- miR:
-
Micro RNA
- MRF:
-
Myogenic regulatory factors
- Myf5:
-
Myogenic factor 5
- MyoD:
-
Myogenic differentiation
- NO:
-
Nitric oxide
- Pax7:
-
Paired box 7
- PCNA:
-
Proliferating cell nuclear antigen
- PGC-1α:
-
Peroxisome proliferator activated receptor gamma coactivator 1 alpha
- PP2CA:
-
Protein phosphatase 2 catalytic subunit, alpha isoform
- PTPN3:
-
Protein tyrosine phosphatase, non-receptor type 3
- ROS:
-
Reactive oxygen species
- TNF:
-
Tumor necrosis factor
- VEGF:
-
Vascular endothelial growth factor
References
Beaudry M, Hidalgo M, Launay T, Bello V, Darribere T (2016) Regulation of myogenesis by environmental hypoxia. J Cell Sci 129:2887–2896. https://doi.org/10.1242/jcs.188904
Bloor CM (2005) Angiogenesis during exercise and training Angiogenesis 8:263–271. https://doi.org/10.1007/s10456-005-9013-x
Britto FA et al (2020) Acute environmental hypoxia potentiates satellite cell-dependent myogenesis in response to resistance exercise through the inflammation pathway in human. FASEB J 34:1885–1900. https://doi.org/10.1096/fj.201902244R
Chaillou T, Lanner JT (2016) Regulation of myogenesis and skeletal muscle regeneration: effects of oxygen levels on satellite cell activity. FASEB J 30:3929–3941. https://doi.org/10.1096/fj.201600757R
Chang NC, Rudnicki MA (2014) Satellite cells: the architects of skeletal muscle. Curr Top Dev Biol 107:161–181. https://doi.org/10.1016/B978-0-12-416022-4.00006-8
Christov C et al (2007) Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell 18:1397–1409. https://doi.org/10.1091/mbc.e06-08-0693
D’Hulst G (1985) Deldicque L (2017) Human skeletal muscle wasting in hypoxia: a matter of hypoxic dose? J Appl Physiol 122:406–408. https://doi.org/10.1152/japplphysiol.00264.2016
Deldicque L, Francaux M (2013) Acute vs chronic hypoxia: what are the consequences for skeletal muscle mass? Cellular and Molecular Exercise Physiology. https://doi.org/10.7457/cmep.v2i1.e5
Di Carlo A, De Mori R, Martelli F, Pompilio G, Capogrossi MC, Germani A (2004) Hypoxia inhibits myogenic differentiation through accelerated MyoD degradation. J Biol Chem 279:16332–16338. https://doi.org/10.1074/jbc.M313931200
Diaz-Ruiz A, Gonzalez-Freire M, Ferrucci L, Bernier M, de Cabo R (2015) SIRT1 synchs satellite cell metabolism with stem cell fate Cell Stem Cell 16:103–104 doi:https://doi.org/10.1016/j.stem.2015.01.006
Doria C et al (2011) Improved VO2 uptake kinetics and shift in muscle fiber type in high-altitude trekkers. J Appl Physiol 111:1597–1605. https://doi.org/10.1152/japplphysiol.01439.2010
Douglas J, Pearson S, Ross A, McGuigan M (2017) Eccentric exercise: physiological characteristics and acute responses. Sports Med 47:663–675. https://doi.org/10.1007/s40279-016-0624-8
Favier FB, Britto FA, Freyssenet DG, Bigard XA, Benoit H (2015) HIF-1-driven skeletal muscle adaptations to chronic hypoxia: molecular insights into muscle physiology. Cell Mol Life Sci 72:4681–4696. https://doi.org/10.1007/s00018-015-2025-9
Fortini P et al (2016) The fine tuning of metabolism, autophagy and differentiation during in vitro myogenesis. Cell Death Dis 7:e2168. https://doi.org/10.1038/cddis.2016.50
Francaux M, Deldicque L (2019) Exercise and the control of muscle mass in human. Pflugers Arch 471:397–411. https://doi.org/10.1007/s00424-018-2217-x
Gnimassou O et al (2018) Environmental hypoxia favors myoblast differentiation and fast phenotype but blunts activation of protein synthesis after resistance exercise in human skeletal muscle. FASEB J 32:5272–5284. https://doi.org/10.1096/fj.201800049RR
Hoppeler H, Kleinert E, Schlegel C, Claassen H, Howald H, Kayar SR, Cerretelli P (1990) Morphological adaptations of human skeletal muscle to chronic hypoxia. Int J Sports Med 11(Suppl 1):S3-9. https://doi.org/10.1055/s-2007-1024846
Hyldahl RD, Olson T, Welling T, Groscost L, Parcell AC (2014) Satellite cell activity is differentially affected by contraction mode in human muscle following a work-matched bout of exercise. Front Physiol 5:485. https://doi.org/10.3389/fphys.2014.00485
Imaoka Y, Kawai M, Mori F, Miyata H (2015) Effect of eccentric contraction on satellite cell activation in human vastus lateralis muscle. J Physiol Sci 65:461–469. https://doi.org/10.1007/s12576-015-0385-4
Konig J, Ott C, Hugo M, Jung T, Bulteau AL, Grune T, Hohn A (2017) Mitochondrial contribution to lipofuscin formation. Redox Biol 11:673–681. https://doi.org/10.1016/j.redox.2017.01.017
Liu W, Wen Y, Bi P, Lai X, Liu XS, Liu X, Kuang S (2012) Hypoxia promotes satellite cell self-renewal and enhances the efficiency of myoblast transplantation. Development 139:2857–2865. https://doi.org/10.1242/dev.079665
Mancinelli R, Pietrangelo T, La Rovere R, Toniolo L, Fano G, Reggiani C, Fulle S (2011) Cellular and molecular responses of human skeletal muscle exposed to hypoxic environment. J Biol Regul Homeost Agents 25:635–645
Mancinelli R, Di Filippo ES, Verratti V, Fulle S, Toniolo L, Reggiani C, Pietrangelo T (2016) The regenerative potential of female skeletal muscle upon hypobaric hypoxic exposure. Front Physiol 7:303. https://doi.org/10.3389/fphys.2016.00303
Manimmanakorn A, Hamlin MJ, Ross JJ, Taylor R, Manimmanakorn N (2013) Effects of low-load resistance training combined with blood flow restriction or hypoxia on muscle function and performance in netball athletes. J Sci Med Sport 16:337–342. https://doi.org/10.1016/j.jsams.2012.08.009
Martin-Rincon M, Morales-Alamo D, Calbet JAL (2018) Exercise-mediated modulation of autophagy in skeletal muscle. Scand J Med Sci Sports 28:772–781. https://doi.org/10.1111/sms.12945
Martinelli M, Winterhalder R, Cerretelli P, Howald H, Hoppeler H (1990) Muscle lipofuscin content and satellite cell volume is increased after high altitude exposure in humans. Experientia 46:672–676. https://doi.org/10.1007/BF01939930
Masschelein E, Van Thienen R, D'Hulst G, Hespel P, Thomis M, Deldicque L (2014) Acute environmental hypoxia induces LC3 lipidation in a genotype-dependent manner FASEB J 28:1022–1034 doi:https://doi.org/10.1096/fj.13-239863
Millet GP, Roels B, Schmitt L, Woorons X, Richalet JP (2010) Combining hypoxic methods for peak performance. Sports Med 40:1–25. https://doi.org/10.2165/11317920-000000000-00000
Mukai K, Ohmura H, Matsui A, Aida H, Takahashi T, Jones JH (2020) High-intensity training in normobaric hypoxia enhances exercise performance and aerobic capacity in Thoroughbred horses: a randomized crossover study. Physiol Rep 8:e14442. https://doi.org/10.14814/phy2.14442
Nagahisa H, Miyata H (2018) Influence of hypoxic stimulation on angiogenesis and satellite cells in mouse skeletal muscle. PLoS ONE 13:e0207040. https://doi.org/10.1371/journal.pone.0207040
Nagahisa H, Mukai K, Ohmura H, Takahashi T, Miyata H (2016) Effect of high-intensity training in normobaric hypoxia on thoroughbred skeletal muscle. Oxid Med Cell Longev 2016:1535367. https://doi.org/10.1155/2016/1535367
Nishimura A, Sugita M, Kato K, Fukuda A, Sudo A, Uchida A (2010) Hypoxia increases muscle hypertrophy induced by resistance training. Int J Sports Physiol Perform 5:497–508. https://doi.org/10.1123/ijspp.5.4.497
Okabe K, Mukai K, Ohmura H, Takahashi T, Miyata H (2017) Effect of acute high-intensity exercise in normobaric hypoxia on Thoroughbred skeletal muscle. J Sports Med Phys Fitness 57:711–719. https://doi.org/10.23736/S0022-4707.16.06154-5
Parise G, Murrant CL, Cocks M, Snijders T, Baum O, Plyley MJ (2020) Capillary facilitation of skeletal muscle function in health and disease. Appl Physiol Nutr Metab 45:453–462. https://doi.org/10.1139/apnm-2019-0416
Park SS, Seo Y-K, Kwon K-S (2019) Sarcopenia targeting with autophagy mechanism by exercise. BMB Rep 52:64–69. https://doi.org/10.5483/BMBRep.2019.52.1.292
Rhoads RP et al (2009) Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway. Am J Physiol Cell Physiol 296:C1321-1328. https://doi.org/10.1152/ajpcell.00391.2008
Roth SM, Martel GF, Ivey FM, Lemmer JT, Metter EJ, Hurley BF, Rogers MA (2000) Skeletal muscle satellite cell populations in healthy young and older men and women. Anatom Record 260:351–358. https://doi.org/10.1002/1097-0185(200012)260:4%3c350::Aid-ar30%3e3.0.Co;2-6
Schwalm C et al (2015) Activation of autophagy in human skeletal muscle is dependent on exercise intensity and AMPK activation. FASEB J 29:3515–3526. https://doi.org/10.1096/fj.14-267187
Scott BR, Slattery KM, Sculley DV, Dascombe BJ (2014) Hypoxia and resistance exercise: a comparison of localized and systemic methods. Sports Med 44:1037–1054. https://doi.org/10.1007/s40279-014-0177-7
Sutton JR (1977) Effect of acute hypoxia on the hormonal response to exercise. J Appl Physiol Respir Environ Exerc Physiol 42:587–592. https://doi.org/10.1152/jappl.1977.42.4.587
Tang AH, Rando TA (2014) Induction of autophagy supports the bioenergetic demands of quiescent muscle stem cell activation. The EMBO Journal 33:2782–2797. https://doi.org/10.15252/embj.201488278
Thomas DP, Fregin GF (1981) Cardiorespiratory and metabolic responses to treadmill exercise in the horse. J Appl Physiol Respir Environ Exerc Physiol 50:864–868. https://doi.org/10.1152/jappl.1981.50.4.864
Tierney MT et al (2014) STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat Med 20:1182–1186. https://doi.org/10.1038/nm.3656
Wei X, Luo L, Chen J (2019) Roles of mTOR Signaling in Tissue Regeneration Cells 8 doi:https://doi.org/10.3390/cells8091075
Wen X, Klionsky DJ (2016) Autophagy is a key factor in maintaining the regenerative capacity of muscle stem cells by promoting quiescence and preventing senescence. Autophagy 12:617–618. https://doi.org/10.1080/15548627.2016.1158373
Yun Z, Lin Q, Giaccia AJ (2005) Adaptive myogenesis under hypoxia STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Mol Cell Biol 25:3040–3055. https://doi.org/10.1128/MCB.25.8.3040-3055.2005
Acknowledgements
MF is supported by the Sports Ministry of the Brussels-Wallonia Federation and the Sports Performance Assistance Centre (SPAC).
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
S.v.D.d.t.R. wrote the first draft, drew the figure and made the summarizing table. L.D. and M.F. corrected the draft and L.D. wrote the final version that was approved by M.F. and S.v.D.d.t.R. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Michael Lindinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
van Doorslaer de ten Ryen, S., Francaux, M. & Deldicque, L. Regulation of satellite cells by exercise in hypoxic conditions: a narrative review. Eur J Appl Physiol 121, 1531–1542 (2021). https://doi.org/10.1007/s00421-021-04641-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-021-04641-4