Abstract
Purpose
The purpose of this single-blind, repeated measures study was to investigate the effect of two hypoxic patterns, continuous or intermittent on key markers of haematological adaptation, stress and cardiac damage in healthy senior participants.
Methods
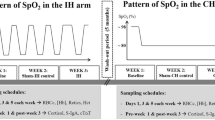
Fifteen healthy senior participants each followed a three-phase protocol over 3 consecutive weeks: (1) 5 consecutive days of breathing room air without a mask (2) 5 days of normoxic mask breathing (sham, FiO2 = 21%) (3) 5 days of intermittent hypoxia (IH) tailored to achieve a mean peripheral oxygen saturation (SpO2) of 85% during ~ 70 min of cumulative exposure to hypoxia. After a 5-month washout period, participants were recalled to undertake continuous hypoxia (CH, SpO2 = 85%, ~ 70 min). The red blood cell count (RBCc), haemoglobin concentration ([Hb]), haematocrit (Hct), percentage of reticulocytes (% Retics), secretory immunoglobulin A (S-IgA), cortisol, cardiac troponin T (cTnT) and the OFF-score (i.e. \(\left[\mathrm{H}\mathrm{b}\right]\bullet 10-60\bullet \sqrt{\% \mathrm{R}\mathrm{e}\mathrm{t}\mathrm{i}\mathrm{c}\mathrm{s}}\)) were measured.
Results
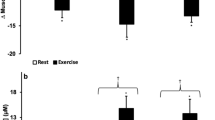
RBCc only increased by day 5 of IH treatment compared to day 5 baseline values (+ 7.7%, p < 0.01) and day 5 Sham values (+ 12.9%, p < 0.01). [Hb] only increased by day 5 of IH treatment compared to day 5 baseline values (+ 14.7%, p < 0.01) and day 5 Sham values (+ 14.3%, p < 0.01). Hct (+ 12.7%, p < 0.01) and the OFF-score (p < 0.05) increased only during the final day of IH treatment. No difference was observed in S-IgA, cortisol or cTnT following IH or CH.
Conclusion
These results revealed that inherent differences in the IH and CH hypoxic patterns could provide crucial components required to trigger hematological changes in senior individuals, without eliciting immunological stress responses or damaging the myocardium.





Similar content being viewed by others
Abbreviations
- CH:
-
Continuous hypoxia
- cTnT:
-
Cardiac troponin T
- EPO:
-
Erythropoietin
- FiO2 :
-
Fraction of inspired oxygen
- Hct:
-
Haematocrit
- [Hb]:
-
Haemoglobin concentration
- HIF:
-
Hypoxia-inducible factors
- IH:
-
Intermittent hypoxia
- PiO2 :
-
Inspired oxygen partial pressure
- RBCc :
-
Red blood cell count
- % Retics:
-
Percentage of reticulocytes
- S-IgA:
-
Secretory immunoglobulin A
- SpO2 :
-
Peripheral oxygen saturation
References
Beidleman BA, Muza SR, Fulco CS, Cymerman A, Staab JE, Sawka MN, Lewis SF, Skrinar GS (2006) White blood cell and hormonal responses to 4300 m altitude before and after intermittent altitude exposure. Clin Sci 111(2):163–169
Burtscher M (2013) Exercise limitations by the oxygen delivery and utilization systems in aging and disease: coordinated adaptation and deadaptation of the lung-heart muscle axis—a mini-review. Gerontology 59(4):289–296. https://doi.org/10.1159/000343990
Burtscher M, Pachinger O, Ehrenbourg I, Mitterbauer G, Faulhaber M, Puhringer R, Tkatchouk E (2004) Intermittent hypoxia increases exercise tolerance in elderly men with and without coronary artery disease. Int J Cardiol 96(2):247–254
Butcher SJ, Jones RL, Eves ND, Petersen SR (2006) Work of breathing is increased during exercise with the self-contained breathing apparatus regulator. Appl Physiol Nutr Metab 31(6):693–701
Costalat G, Lemaitre F, Tobin B, Renshaw G (2017) Intermittent hypoxia revisited: a promising non-pharmaceutical strategy to reduce cardio-metabolic risk factors? Sleep Breath 2(10):17–1459
Dharmarajan TS, Avula S, Norkus EP (2006) Anemia increases risk for falls in hospitalized older adults: an evaluation of falls in 362 hospitalized, ambulatory, long-term care, and community patients. J Am Med Dir Assoc 7(5):287–293
Di Giulio C, Bianchi G, Cacchio M, Artese L, Piccirilli M, Verratti V, Valerio R, Iturriaga R (2006) Neuroglobin, a new oxygen binding protein is present in the carotid body and increases after chronic intermittent hypoxia. In: Hayashida Y, Gonzalez C, Kondo H (eds) The arterial chemoreceptors. Advances in experimental medicine ands biology, vol 580. Springer, Boston
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37(2):247–248
Dubois AB, Botelho SY, Comroe JH Jr (1956) A new method for measuring airway resistance in man using a body plethysmograph: values in normal subjects and in patients with respiratory disease. J Clin Investig 35(3):327–335. https://doi.org/10.1172/jci103282
Eckardt KU, Boutellier U, Kurtz A, Schopen M, Koller EA, Bauer C (1989) Rate of erythropoietin formation in humans in response to acute hypobaric hypoxia. J Appl Physiol 66(4):1785–1788. https://doi.org/10.1152/jappl.1989.66.4.1785
Gore CJ, Parisotto R, Ashenden MJ, Stray-Gundersen J, Sharpe K, Hopkins W, Emslie KR, Howe C, Trout GJ, Kazlauskas R, Hahn AG (2003) Second-generation blood tests to detect erythropoietin abuse by athletes. Haematologica 88(3):333–344
Haase VH (2013) Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev 27(1):41–53. https://doi.org/10.1016/j.blre.2012.12.003
Harshman SW, Geier BA, Fan M, Rinehardt S, Watts BS, Drummond LA, Preti G, Phillips JB, Ott DK, Grigsby CC (2015) The identification of hypoxia biomarkers from exhaled breath under normobaric conditions. J Breath Res 9(4):1752–7155
Hauser A, Troesch S, Saugy JJ, Schmitt L, Cejuela-Anta R, Faiss R, Steiner T, Robinson N, Millet GP, Wehrlin JP (2017) Individual hemoglobin mass response to normobaric and hypobaric "live high-train low": a one-year crossover study. J Appl Physiol 123(2):387–393
Izaks GJ, Westendorp RG, Knook DL (1999) The definition of anemia in older persons. JAMA 281(18):1714–1717
Jelkmann W (2011) Regulation of erythropoietin production. J Physiol 589(Pt 6):1251–1258
Khanna K, Mishra KP, Ganju L, Kumar B, Singh SB (2018) High-altitude-induced alterations in gut-immune axis: a review. Int Rev Immunol 37(2):119–126. https://doi.org/10.1080/08830185.2017.1407763
Kim TS, Hanak M, Trampont PC, Braciale TJ (2015) Stress-associated erythropoiesis initiation is regulated by type 1 conventional dendritic cells. J Clin Investig 125(10):3965–3980
Levine BD, Stray-Gundersen J (1997) "Living high-training low": effect of moderate-altitude acclimatization with low-altitude training on performance. J Appl Physiol 83(1):102–112
Lobigs LM, Sharpe K, Garvican-Lewis LA, Gore CJ, Peeling P, Dawson B, Schumacher YO (2017) The athlete's hematological response to hypoxia: a meta-analysis on the influence of altitude exposure on key biomarkers of erythropoiesis. Am J Hematol 93(1):74–83. https://doi.org/10.1002/ajh.24941
Locatelli F, Del Vecchio L (2014) Haemoglobin levels and health-related quality of life: a neglected hard end point. Nephrol Dial Transplant 29(7):1272–1274. https://doi.org/10.1093/ndt/gfu059
Lucca U, Tettamanti M, Mosconi P, Apolone G, Gandini F, Nobili A, Tallone MV, Detoma P, Giacomin A, Clerico M, Tempia P, Guala A, Fasolo G, Riva E (2008) Association of mild anemia with cognitive, functional, mood and quality of life outcomes in the elderly: the “Health and Anemia” study. PLoS ONE 3(4):e1920. https://doi.org/10.1371/journal.pone.0001920
Lundby C, Millet GP, Calbet JA, Bartsch P, Subudhi AW (2012) Does 'altitude training' increase exercise performance in elite athletes? Br J Sports Med 46(11):792–795
Mackenzie R, Maxwell N, Castle P, Brickley G, Watt P (2011) Acute hypoxia and exercise improve insulin sensitivity (SI2*) in individuals with type 2 diabetes. Diabetes Metab Res Rev 27(1):94–101. https://doi.org/10.1002/dmrr.1156
Michiels C, Tellier C, Feron O (2016) Cycling hypoxia: a key feature of the tumor microenvironment. Biochimica et Biophysica Acta Rev Cancer 1:76–86. https://doi.org/10.1016/j.bbcan.2016.06.004
Millet GP, Brocherie F, Girard O, Wehrlin JP, Troesch S, Hauser A, Steiner T, Peltonen JE, Rusko HK, Constantini K, Fulton TJ, Hursh DG, Noble TJ, Paris HL, Wiggins CC, Chapman RF, Levine BD, Kumar VH, Schmidt WF (2016) Commentaries on viewpoint: time for a new metric for hypoxic dose ? J Appl Physiol (1985) 121(1):356–358. https://doi.org/10.1152/japplphysiol.00460.2016
Montero D, Lundby C (2019) Arterial oxygen content regulates plasma erythropoietin independent of arterial oxygen tension: a blinded crossover study. Kidney Int 95(1):173–177. https://doi.org/10.1016/j.kint.2018.09.015
Muza SR, Beidleman BA, Fulco CS (2010) Altitude preexposure recommendations for inducing acclimatization. High Alt Med Biol 11(2):87–92
Navarrete-Opazo A, Mitchell GS (2014) Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol 307(10):R1181–R1197
Pottgiesser T, Sottas PE, Echteler T, Robinson N, Umhau M, Schumacher YO (2011) Detection of autologous blood doping with adaptively evaluated biomarkers of doping: a longitudinal blinded study. Transfusion 51(8):1707–1715
Ramos-Campo DJ, Martinez-Sanchez F, Esteban-Garcia P, Rubio-Arias JA, Clemente-Suarez VJ, Jimenez-Diaz JF (2015) The effects of intermittent hypoxia training on hematological and aerobic performance in triathletes. Acta Physiol Hung 102(4):409–418
Renshaw G, Nikinmaa M (2007) Oxygen sensors of the peripheral and central nervous system. In: Johnson D (ed) Handbook of neurochemistry and molecular neurobiology, vol 20. Springer, New York
Richalet JP, Gore CJ (2008) Live and/or sleep high:train low, using normobaric hypoxia. Scand J Med Sci Sports 1:29–37
Rodriguez FA, Iglesias X, Feriche B, Calderon-Soto C, Chaverri D, Wachsmuth NB, Schmidt W, Levine BD (2015) Altitude training in elite swimmers for sea level performance (Altitude Project). Med Sci Sports Exerc 47(9):1965–1978
Schmidt W, Prommer N (2010) Impact of alterations in total hemoglobin mass on VO2max. Exerc Sport Sci Rev 38(2):68–75
Schütz F, Zollinger A (2018) ABPS: an R package for calculating the abnormal blood profile score. Front Physiol 9:1638
Serebrovskaya TV (2002) Intermittent hypoxia research in the former soviet union and the commonwealth of independent states: history and review of the concept and selected applications. High Alt Med Biol 3(2):205–221
Serebrovskaya TV, Nosar VI, Bratus LV, Gavenauskas BL, Mankovska IM (2013) Tissue oxygenation and mitochondrial respiration under different modes of intermittent hypoxia. High Alt Med Biol 14(3):280–288
Shatilo VB, Korkushko OV, Ischuk VA, Downey HF, Serebrovskaya TV (2008) Effects of intermittent hypoxia training on exercise performance, hemodynamics, and ventilation in healthy senior men. High Alt Med Biol 9(1):43–52
Torpel A, Peter B, Hamacher D, Schega L (2019) Dose-response relationship of intermittent normobaric hypoxia to stimulate erythropoietin in the context of health promotion in young and old people. Eur J Appl Physiol 11(10):019–04096
Turner G, Gibson OR, Watt PW, Pringle JSM, Richardson AJ, Maxwell NS (2017) The time course of endogenous erythropoietin, IL-6, and TNFalpha in response to acute hypoxic exposures. Scand J Med Sci Sports 27(7):714–723
Verges S, Chacaroun S, Godin-Ribuot D, Baillieul S (2015) Hypoxic conditioning as a new therapeutic modality. Front Pediatr. https://doi.org/10.3389/fped.2015.00058
Wahl P, Schmidt A, Achtzehn S, Bloch W, Mester J (2013) Responses of angiogenic growth factors to exercise, to hypoxia and to exercise under hypoxic conditions. Int J Sports Med 34(02):95–100
Wang JS, Chen LY, Fu LL, Chen ML, Wong MK (2007) Effects of moderate and severe intermittent hypoxia on vascular endothelial function and haemodynamic control in sedentary men. Eur J Appl Physiol 100(2):127–135
Wang Y, Liu X, Xie B, Yuan H, Zhang Y, Zhu J (2020) The NOTCH1-dependent HIF1α/VGLL4/IRF2BP2 oxygen sensing pathway triggers erythropoiesis terminal differentiation. Redox Biol 28:101313. https://doi.org/10.1016/j.redox.2019.101313
Zamboni V, Cesari M, Zuccala G, Onder G, Woodman RC, Maraldi C, Ranzini M, Volpato S, Pahor M, Bernabei R (2006) Anemia and cognitive performance in hospitalized older patients: results from the GIFA study. Int J Geriatr Psychiatry 21(6):529–534
Acknowledgements
The authors would like to thank Biomedtech Australia Pty. Ltd., the Heart Foundation Research Centre Griffith University, and the Griffith Health Institute for generously supporting this independent investigation. We greatly appreciated the opportunity to borrow hypoxicators from Oleg Bassovitch, Biomedtech (GO2Altitude®). We are grateful to the participants for their enthusiastic involvement in this study, to Taryn Mann for quantifying troponin T to Dr. Clare Minahan and Dr. Mike Steele for statistical advice as well as to Dr. Glenn Harrison for insightful comments on drafts of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there was no conflict of interest and that the results of the present study do not constitute endorsement of any specific equipment for hypoxia delivery.
Additional information
Communicated by Susan Hopkins.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tobin, B., Costalat, G. & Renshaw, G.M.C. Intermittent not continuous hypoxia provoked haematological adaptations in healthy seniors: hypoxic pattern may hold the key. Eur J Appl Physiol 120, 707–718 (2020). https://doi.org/10.1007/s00421-020-04310-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-020-04310-y