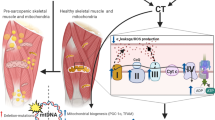
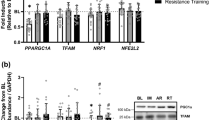
Abstract
Mitochondria are responsible for aerobic respiration and large-scale ATP production in almost all cells of the body. Their function is decreased in many neurodegenerative and cardiovascular disease states, in metabolic disorders such as type II diabetes and obesity, and as a normal component of aging. Disuse of skeletal muscle from immobilization or unloading triggers alterations of mitochondrial density and activity. Resultant mitochondrial dysfunction after paralysis, which precedes muscle atrophy, may augment subsequent release of reactive oxygen species leading to protein ubiquitination and degradation. Spinal cord injury is a unique form of disuse atrophy as there is a complete or partial disruption in tonic communication between the central nervous system (CNS) and skeletal muscle. Paralysis, unloading and disruption of CNS communication result in a rapid decline in skeletal muscle function and metabolic status with disruption in activity of peroxisome-proliferator-activated receptor-gamma co-activator 1 alpha and calcineurin, key regulators of mitochondrial health and function. External interventions, both acute and chronical with training using body-weight-assisted treadmill training or electrical stimulation have consistently demonstrated adaptations in skeletal muscle mitochondria, and expression of the genes and proteins required for mitochondrial oxidation of fats and carbohydrates to ATP, water, and carbon dioxide. The purpose of this mini-review is to highlight our current understanding as to how paralysis mechanistically triggers downstream regulation in mitochondrial density and activity and to discuss how mitochondrial dysfunction may contribute to skeletal muscle atrophy.







Similar content being viewed by others
References
Abadi A, Glover EI, Isfort RJ, Raha S, Safdar A, Yasuda N, Kaczor JJ, Melov S, Hubbard A, Qu X, Phillips SM, Tarnopolsky M (2009) Limb immobilization induces a coordinate down-regulation of mitochondrial and other metabolic pathways in men and women. PLoS One 4(8):e6518
Adhihetty PJ, Ljubicic V, Menzies KJ, Hood DA (2005) Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. Am J Physiol Cell Physiol 289(4):C994–C1001
Adhihetty PJ, O’Leary MF, Chabi B, Wicks KL, Hood DA (2007) Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol 102(3):1143–1151
Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, Xie W, Shiomi T, Zalk R, Lacampagne A, Marks AR (2011) Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab 14(2):196–207
Arija-Blázquez A, Ceruelo-Abajo S, Díaz-Merino MS, Godino-Durán JA, Martínez-Dhier L, Martin JL, Florensa-Vila J (2014) Effects of electromyostimulation on muscle and bone in men with acute traumatic spinal cord injury: a randomized clinical trial. J Spinal Cord Med 37(3):299–309
Bank M, Stein A, Sison C, Glazer A, Jassal N, McCarthy D, Shatzer M, Hahn B, Chugh R, Davies P, Bloom O (2015) Elevated circulating levels of the pro-inflammatory cytokine macrophage migration inhibitory factor in individuals with acute spinal cord injury. Arch Phys Med Rehabil 96(4):633–644
Bauman WA, Spungen AM (2001) Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med 24(4):266–277
Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R (1996) The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med 335(1):1–7
Bhattacharya A, Muller FL, Liu Y, Sabia M, Liang H, Song W, Jang YC, Ran Q, Van Remmen H (2009) Denervation induces cytosolic phospholipase A2-mediated fatty acid hydroperoxide generation by muscle mitochondria. J Biol Chem 284(1):46–55
Bhattacharya A, Lustgarten M, Shi Y, Liu Y, Jang YC, Pulliam D, Jernigan AL, Van Remmen H (2011) Increased mitochondrial matrix-directed superoxide production by fatty acid hydroperoxides in skeletal muscle mitochondria. Free Radic Biol Med 50(5):592–601
Bodine SC (2013) Disuse-induced muscle wasting. Int J Biochem Cell Biol 45(10):2200–2208
Bonaldo P, Sandri M (2013) Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech 6(1):25–39
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287(4):C817–C833
Brown JL, Rosa-Caldwell ME, Lee DE, Blackwell TA, Brown LA, Perry RA, Haynie WS, Hardee JP, Carson JA, Wiggs MP, Washington TA, Greene NP (2017) Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J Cachexia Sarcopenia Muscle 8(6):926–938
Calabria E, Ciciliot S, Moretti I, Garcia M, Picard A, Dyar KA, Pallafacchina G, Tothova J, Schiaffino S, Murgia M (2009) NFAT isoforms control activity-dependent muscle fiber type specification. Proc Natl Acad Sci USA 106(32):13335–13340
Cartee GD, Hepple RT, Bamman MM, Zierath JR (2016) Exercise promotes healthy aging of skeletal muscle. Cell Metab 23(6):1034–1047
Carvalho de Abreu DC, Júnior AC, Rondina JM, Cendes F (2008) Muscle hypertrophy in quadriplegics with combined electrical stimulation and body weight support training. Int J Rehabil Res 31(2):171–175
Cea LA, Cisterna BA, Puebla C, Frank M, Figueroa XF, Cardozo C, Willecke K, Latorre R, Sáez JC (2013) De novo expression of connexin hemichannels in denervated fast skeletal muscles leads to atrophy. Proc Natl Acad Sci USA 110(40):16229–16234
Chan DC (2006) Mitochondria: dynamic organelles in disease, aging, and development. Cell 125(7):1241–1252
Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC (2010) Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141(2):280–289
Chilibeck PD, Bell G, Jeon J, Weiss CB, Murdoch G, MacLean I, Ryan E, Burnham R (1999) Functional electrical stimulation exercise increases GLUT-1 and GLUT-4 in paralyzed skeletal muscle. Metabolism 48(11):1409–1413
Cirnigliaro CM, LaFountaine MF, Dengel DR, Bosch TA, Emmons RR, Kirshblum SC, Sauer S, Asselin P, Spungen AM, Bauman WA (2015) Visceral adiposity in persons with chronic spinal cord injury determined by dual energy X-ray absorptiometry. Obesity (Silver Spring Md) 23(9):1811–1817
Cogswell AM, Stevens RJ, Hood DA (1993) Properties of skeletal muscle mitochondria isolated from subsarcolemmal and intermyofibrillar regions. Am J Physiol 264(2 Pt1):C383–C389
Coughlan KA, Valentine RJ, Ruderman NB, Saha AK (2014) AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes 7:241–253
Crossland H, Kazi AA, Lang CH, Timmons JA, Pierre P, Wilkinson DJ, Smith K, Szewczyk NJ, Atherton PJ (2013) Focal adhesion kinase is required for IGF-I-mediated growth of skeletal muscle cells via a TSC2/ mTOR/S6K1-associated pathway. Am J Physiol Endocrinol Metab 305(2):E183–E193
Davies AL, Hayes KC, Dekaban GA (2007) Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil 88(11):1384–1393
de Abreu DC, Cliquet A Jr, Rondina JM, Cendes F (2009) Electrical stimulation during gait promotes increase of muscle cross-sectional area in quadriplegics: a preliminary study. Clin Orthop Relat Res 467(2):553–557
Dodd KM, Tee AR (2012) Leucine and mTORC1: a complex relationship. Am J Physiol Endocrinol Metab 302(11):E1329–E1342
Dudley GA, Castro MJ, Rogers S, Apple DF Jr (1999) A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol 80(4):394–396
Durieux AC, D’Antona G, Desplanches D, Freyssenet D, Klossner S, Bottinelli R, Flück M (2009) Focal adhesion kinase is a load-dependent governor of the slow contractile and oxidative muscle phenotype. J Physiol 587(Pt 14):3703–3717
Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA (2004) Intramuscular fat and glucose tolerance after spinal cord injury-a cross-sectional study. Spinal Cord 42(12):711–716
Erickson ML, Ryan TE, Young HJ, McCully KK (2013) Near-infrared assessments of skeletal muscle oxidative capacity in persons with spinal cord injury. Eur J Appl Physiol 113(9):2275–2283
Erickson ML, Ryan TE, Backus D, McCully KK (2017) Endurance neuromuscular electrical stimulation training improves skeletal muscle oxidative capacity in individuals with motor-complete spinal cord injury. Muscle Nerve 55(5):669–675
Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR (1996) Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol Endocrinol Metab 270(4 Pt1):E627–E633
Garton FC, Seto JT, Quinlan KG, Yang N, Houweling PJ, North KN (2014) Alpha-Actinin-3 deficiency alters muscle adaptation in response to denervation and immobilization. Hum Mol Genet 23(7):1879–1893
Giangregorio LM, Hicks AL, Webber CE, Phillips SM, Craven BC, Bugaresti JM, McCartney N (2005) Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal Cord 43(11):649–657
Giangregorio LM, Webber CE, Phillips SM, Hicks AL, Craven BC, Bugaresti JM, McCartney N (2006) Can body weight supported treadmill training increase bone mass and reverse muscle atrophy in individuals with chronic incomplete spinal cord injury? Appl Physiol Nutr Metab 31(3):283–291
Giangregorio L, Craven C, Richards K, Kapadia N, Hitzig SL, Masani K, Popovic MR (2012) A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: effects on body composition. J Spinal Cord Med 35(5):351–360
Glancy B, Hartnell LM, Malide D, Yu ZX, Combs CA, Connelly PS, Subramaniam S, Balaban RS (2015) Mitochondrial reticulum for cellular energy distribution in muscle. Nature 523(7562):617–620
Glover EI, Yasuda N, Tarnopolsky MA, Abadi A, Phillips SM (2010) Little change in markers of protein breakdown and oxidative stress in humans in immobilization-induced skeletal muscle atrophy. Appl Physiol Nutr Metab 35(2):125–133
Goldmann WH (2014) Mechanosensation: a basic cellular process. Prog Mol Biol Transl Sci 126:75–102
Gorgey AS, Dudley GA (2007) Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord 45(4):304–309
Gorgey AS, Lawrence J (2016) Acute responses of functional electrical stimulation cycling on the ventilation-to-CO2 production ratio and substrate utilization after spinal cord injury. PM R 8(3):225–234
Gorgey AS, Shepherd C (2010) Skeletal muscle hypertrophy and decreased intramuscular fat after unilateral resistance training in spinal cord injury: case report. J Spinal Cord Med 33(1):90–95
Gorgey AS, Mather KJ, Cupp HR, Gater DR (2012) Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc 44(1):165–174
Gorgey AS, Graham ZA, Bauman WA, Cardozo C, Gater DR (2017) Abundance in proteins expressed after functional electrical stimulation cycling or arm cycling ergometry training in persons with chronic spinal cord injury. J Spinal Cord Med 40(4):439–448
Gouspillou G, Sgarioto N, Kapchinsky S, Purves-Smith F, Norris B, Pion CH, Barbat-Artigas S, Lemieux F, Taivassalo T, Morais JA, Aubertin-Leheudre M, Hepple RT (2014) Increased sensitivity to mitochondrial permeability transition and myonuclear translocation of endonuclease G in atrophied muscle of physically active older humans. FASEB J 28(4):1621–1633
Graham ZA, Qin W, Harlow LC, Ross NH, Bauman WA, Gallagher PM, Cardozo CP (2016) Focal adhesion kinase signaling is decreased 56 days following spinal cord injury in rat gastrocnemius. Spinal Cord 54:502–509
Graham ZA, Harlow L, Bauman WA, Cardozo CP (2018) Alterations in mitochondrial fission, fusion, and mitophagic protein expression in the gastrocnemius of mice after a sciatic nerve transection. Muscle Nerve 58(4):592–599
Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A (2007) The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 282(41):30107–30119
Griffin L, Decker MJ, Hwang JY, Wang B, Kitchen K, Ding Z, Ivy JL (2009) Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol 19(4):614–622
Halestrap AP (2009) What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 46(6):821–831
Halestrap AP, Richardson AP (2015) The mitochondrial permeability transition: a current perspective on its identity and role in ischaemia/reperfusion injury. J Mol Cell Cardiol 78:129–141
Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM (2003) An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc Natl Acad Sci USA 100(12):7111–7116
Hepple RT (2016) Impact of aging on mitochondrial function in cardiac and skeletal muscle. Free Radic Biol Med 98:177–186
Hesselink MK, Schrauwen-Hinderling V, Schrauwen P (2016) Skeletal muscle mitochondria as a target to prevent or treat type 2 diabetes mellitus. Nat Rev Endocrinol 12(11):633–645
Hjeltnes N, Galuska D, Björnholm M, Aksnes AK, Lannem A, Zierath JR, Wallberg-Henriksson H (1998) Exercise-induced overexpression of key regulatory proteins involved in glucose uptake and metabolism in tetraplegic persons: molecular mechanism for improved glucose homeostasis. FASEB J 12(15):1701–1712
Hood DA, Tryon LD, Carter HN, Kim Y, Chen CC (2016) Unravelling the mechanisms regulating muscle mitochondrial biogenesis. Biochem J 473(15):2295–2314
Hoppeler H (2016) Molecular networks in skeletal muscle plasticity. J Exp Biol 219(Pt 2):205–213
Ingalls CP, Warren GL, Armstrong RB (1999) Intracellular Ca2 transients in mouse soleus muscle after hindlimb unloading and reloading. J Appl Physiol 87(1):386–390
Invernizzi M, Carda S, Rizzi M, Grana E, Squarzanti DF, Cisari C, Molinari C, Renò F (2015) Evaluation of serum myostatin and sclerostin levels in chronic spinal cord injured patients. Spinal Cord 53(8):615–620
Iqbal S, Ostojic O, Singh K, Joseph A, Hood DA (2013) Expression of mitochondrial fission and fusion regulatory proteins in skeletal muscle during chronic use and disuse. Muscle Nerve 48(6):963–970
Janostiak R, Pataki AC, Brábek J, Rösel D (2014) Mechanosensors in integrin signaling: the emerging role of p130Cas. Eur J Cell Biol 93(10–12):445–454
Jayaraman A, Shah P, Gregory C, Bowden M, Stevens J, Bishop M, Walter G, Behrman A, Vandenborne K (2008) Locomotor training and muscle function after incomplete spinal cord injury: case series. J Spinal Cord Med 31(2):185–193
Jeon JY, Weiss CB, Steadward RD, Ryan E, Burnham RS, Bell G, Chilibeck P, Wheeler GD (2002) Improved glucose tolerance and insulin sensitivity after electrical stimulation-assisted cycling in people with spinal cord injury. Spinal Cord 40(3):110–117
Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ (2009) Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res 104(11):1240–1252
Karam C, Yi J, Xiao Y, Dhakal K, Zhang L, Li X, Manno C, Xu J, Li K, Cheng H, Ma J, Zhou J (2017) Absence of physiological Ca2+ transients is an initial trigger for mitochondrial dysfunction in skeletal muscle following denervation. Skelet Muscle 7(1):6
Kavazis AN, McClung JM, Hood DA, Powers SK (2008) Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli. Am J Physiol Heart Circ Physiol 294(2):H928–H935
Kavazis AN, Talbert EE, Smuder AJ, Hudson MB, Nelson WB, Powers SK (2009) Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radic Biol Med 46(6):842–850
Kern H, Boncompagni S, Rossini K, Mayr W, Fano G, Zanin ME, Podhorska-Okolow M, Protasi F, Carraro U (2004) Long-term denervation in humans causes degeneration of both contractile and excitation-contraction coupling apparatus, which is reversible by functional electrical stimulation (FES): a role for myofiber regeneration? J Neuropathol Exp Neurol 63(9):919–931
Kern H, Hofer C, Modlin M, Mayr W, Vindigni V, Zampieri S, Boncompagni S, Protasi F, Carraro U (2008) Stable muscle atrophy in long-term paraplegics with complete upper motor neuron lesion from 3- to 20-year SCI. Spinal Cord 46(4):293–304
Kim JA, Roy RR, Zhong H, Alaynick WA, Embler E, Jang C, Gomez G, Sonoda T, Evans RM, Edgerton VR (2015) PPAR delta preserves a high resistance to fatigue in the mouse medial gastrocnemius after spinal cord transection. Muscle Nerve 53(2):287–296
Kjaer M, Mohr T, Biering-Sørensen F, Bangsbo J (2001) Muscle enzyme adaptation to training and tapering-off in spinal-cord-injured humans. Eur J Appl Physiol 84(5):482–486
Klossner S, Durieux A-C, Freyssenet D, Flueck M (2009) Mechano- transduction to muscle protein synthesis is modulated by FAK. Eur J Appl 106(3):389–398
Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE (2009) Mitochondria and reactive oxygen species. Free Radic Biol Med 47(4):333–343
Krieger DA, Tate CA, McMillin-Wood J, Booth FW (1980) Populations of rat skeletal muscle mitochondria after exercise and immobilization. J Appl Physiol 48(1):23–28
Laker RC, Xu P, Ryall KA, Sujkowski A, Kenwood BM, Chain KH, Zhang M, Royal MA, Hoehn KL, Driscoll M, Adler PN, Wessells RJ, Saucerman JJ, Yan Z (2014) A novel MitoTimer reporter gene for mitochondrial content, structure, stress, and damage in vivo. J Biol Chem 289(17):12005–12015
Lammers G, Poelkens F, van Duijnhoven NT, Pardoel EM, Hoenderop JG, Thijssen DH, Hopman MT (2012) Expression of genes involved in fatty acid transport and insulin signaling is altered by physical inactivity and exercise training in human skeletal muscle. Am J Physiol Endocrinol Metab 303(10):E1245–E1251
Léger B, Senese R, Al-Khodairy AW, Dériaz O, Gobelet C, Giacobino JP, Russell AP (2009) Atrogin-1, MuRF1, and FoXO, as well as phosphorylated GSK-3beta and 4E-BP1 are reduced in skeletal muscle of chronic spinal cord-injured patients. Muscle Nerve 40(1):69–78
Lesnefsky EJ, Chen Q, Hoppel CL (2016) Mitochondrial metabolism in aging heart. Circ Res 118(10):1593–1611
Lesnefsky EJ, Chen Q, Tandler B, Hoppel CL (2017) Mitochondrial dysfunction and myocardial ischemia-reperfusion: implications for novel therapies. Annu Rev Pharmacol Toxicol 57:535–565
Li YP, Chen Y, Li AS, Reid MB (2003) Hydrogen peroxide stimulates ubiquitin-conjugating activity and expression of genes for specific E2 and E3 proteins in skeletal muscle myotubes. Am J Physiol Cell Physiol 285(4):C806–C812
Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM (2002) Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418(6899):797–801
Liu XH, Harlow L, Graham ZA, Bauman WA, Cardozo C (2017) Spinal cord injury leads to hyperoxidation and nitrosylation of skeletal muscle ryanodine receptor-1 associated with upregulation of nicotinamide adenine dinucleotide phosphate oxidase 4. J Neurotrauma 34(12):2069–2074
Mahoney ET, Bickel CS, Elder C, Black C, Slade JM, Apple D Jr, Dudley GA (2005) Changes in skeletal muscle size and glucose tolerance with electrically stimulated resistance training in subjects with chronic spinal cord injury. Arch Phys Med Rehabil 86(7):1502–1504
Martin-Rincon M, Morales-Alamo D, Calbet JAL (2018) Exercise-mediated modulation of autophagy in skeletal muscle. Scand J Med Sci Sports 28(3):772–781
Marzetti E, Wohlgemuth SE, Lees HA, Chung HY, Giovannini S, Leeuwenburgh C (2008) Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech Ageing Dev 129(9):542–549
Marzetti E, Hwang JC, Lees HA, Wohlgemuth SE, Dupont-Versteegden EE, Carter CS, Bernabei R, Leeuwenburgh C (2010) Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy. Biochim Biophys Acta 1800(3):235–244
Max SR (1972) Disuse atrophy of skeletal muscle: loss of functional activity of mitochondria. Biochem Biophys Res Commun 46:1394–1398
McClung JM, Whidden MA, Kavazis AN, Falk DJ, Deruisseau KC, Powers SK (2008) Redox regulation of diaphragm proteolysis during mechanical ventilation. Am J Physiol Regul Integr Comp Physiol 294:R1608–R1617
McCully KK, Mulcahy TK, Ryan TE, Zhao Q (2011) Skeletal muscle metabolism in individuals with spinal cord injury. J Appl Physiol 111(1):143–148
Min K, Smuder AJ, Kwon OS, Kavazis AN, Szeto HH, Powers SK (2011) Mitochondrial-targeted antioxidants protect the skeletal muscle against immobilization-induced muscle atrophy. J Appl Physiol 111(5):1459–1466
Mohr T, Dela F, Handberg A, Biering-Sørensen F, Galbo H, Kjaer M (2001) Insulin action and long-term electrically induced training in individuals with spinal cord injuries. Med Sci Sports Exerc 33(8):1247–1252
Mounier R, Théret M, Lantier L, Foretz M, Viollet B (2015) Expanding roles for AMPK in skeletal muscle plasticity. Trends Endocrinol Metab 26(6):275–286
Muller FL, Song W, Jang YC, Liu Y, Sabia M, Richardson A, Van Remmen H (2007) Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol Regul Integr Comp Physiol 293(3):R1159–R1168
Naya FJ, Mercer B, Shelton J, Richardson JA, Williams RS, Olson EN (2000) Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J Biol Chem 275(7):4545–4548
O’Brien LC, Chen Q, Savas J, Lesnefsky EJ, Gorgey AS (2017a) Skeletal muscle mitochondrial mass is linked to lipid and metabolic profile in individuals with spinal cord injury. Eur J Appl Physiol 117(11):2137–2147
O’Brien LC, Wade RC, Segal L, Chen Q, Savas J, Lesnefsky EJ, Gorgey AS (2017b) Mitochondrial mass and activity as a function of body composition in individuals with spinal cord injury. Physiol Rep 5(3):e13080
Ohnishi T, Ohnishi ST, Shinzawa-Itoh K, Yoshikawa S, Weber RT (2012) EPR detection of two protein-associated ubiquinone components (SQ(Nf) and SQ(Ns)) in the membrane in situ and in proteoliposomes of isolated bovine heart complex I. Biochim Biophys Acta 1817(10):1803–1809
Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G (2003) Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem 278(10):7743–7746
Papa S (1996) Mitochondrial oxidative phosphorylation changes in the life span. Molecular aspects and physiological implications. Biochim Biophys Acta 1276(2):87–105
Petrie MA, Suneja M, Faidley E, Shields RK (2014) A minimal dose of electrically induced muscle activity regulates distinct gene signaling pathways in humans with spinal cord injury. PLoS One 9(12):e115791
Petrie M, Suneja M, Shields RK (2015) Low-frequency stimulation regulates metabolic gene expression in paralyzed muscle. J Appl Physiol (1985) 118:723–731
Phielix E, Mensink M (2008) Type 2 diabetes mellitus and skeletal muscle metabolic function. Physiol Behav 94(2):252–258
Phillips SM (2009) Physiologic and molecular bases of muscle hypertrophy and atrophy: impact of resistance exercise on human skeletal muscle (protein and exercise dose effects). Appl Physiol Nutr Metab 34(3):403–410
Phillips SM, Stewart BG, Mahoney DJ, Hicks AL, McCartney N, Tang JE, Wilkinson SB, Armstrong D, Tarnopolsky MA (2004) Body-weight-support treadmill training improves blood glucose regulation in persons with incomplete spinal cord injury. J Appl Physiol (1985) 97(2):716–724
Phillips SM, Glover EI, Rennie MJ (2009) Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J Appl Physiol 107(3):645–654
Plant PJ, Bain JR, Correa JE, Woo M, Batt J (2009) Absence of caspase-3 protects against denervation-induced skeletal muscle atrophy. J Appl Physiol (1985) 107(1):224–234
Pollock N, Staunton CA, Vasilaki A, McArdle A, Jackson MJ (2017) Denervated muscle fibers induce mitochondrial peroxide generation in neighboring innervated fibers: role in muscle aging. Free Radic Biol Med 112:84–92
Porter C, Reidy PT, Bhattarai N, Sidossis LS, Rasmussen BB (2015) Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc 47(9):1922–1931
Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ (2011a) Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med 39(7):1749–1759
Powers SK, Ji LL, Kavazis AN, Jackson MJ (2011b) Reactive oxygen species: impact on skeletal muscle. Compr Physiol 1(2):941–969
Powers SK, Smuder AJ, Criswell DS (2011c) Mechanistic links between oxidative stress and disuse muscle atrophy. Antioxid Redox Signal 15(9):2519–2528
Powers SK, Wiggs MP, Duarte JA, Zergeroglu AM, Demirel HA (2012) Mitochondrial signaling contributes to disuse muscle atrophy. Am J Physiol Endocrinol Metab 303(1):E31–E39
Qin W, Pan J, Wu Y, Bauman WA, Cardozo C (2014) Anabolic steroids activate calcineurin-NFAT signaling and thereby increase myotube size and reduce denervation atrophy. Mol Cell Endocrinol 399:336–345
Reid MB, Moylan JS (2011) Beyond atrophy: redox mechanisms of muscle dysfunction in chronic inflammatory disease. J Physiol 589(Pt 9):2171–2179
Rizzuto R, De Stefani D, Raffaello A, Mammucari C (2012) Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13(9):566–578
Rochester L, Barron MJ, Chandler CS, Sutton RA, Miller S, Johnson MA (1995) Influence of electrical stimulation of the tibialis anterior muscle in paraplegic subjects. 2. Morphological and histochemical properties. Paraplegia 33(9):514–522
Romanello V, Sandri M (2010) Mitochondrial biogenesis and fragmentation as regulators of muscle protein degradation. Curr Hypertens Rep 12(6):433–439
Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Del Piccolo P, Foretz M, Scorrano L, Rudolf R, Sandri M (2010) Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J 29(10):1774–1785
Roy RR, Zhong H, Monti RJ, Vallance KA, Edgerton VR (2002) Mechanical properties of the electrically silent adult rat soleus muscle. Muscle Nerve 26:404–412
Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, Lanza IR, Rasbach KA, Okutsu M, Nair KS, Yan Z, Leinwand LA, Spiegelman BM (2012) A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 151(6):1319–1331
Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y (2010) AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab 298(4):E751–E760
Russo TL, Peviani SM, Durigan JL, Gigo-Benato D, Delfino GB, Salvini TF (2010) Stretching and electrical stimulation reduce the accumulation of MyoD, myostatin and atrogin-1 in denervated rat skeletal muscle. J Muscle Res Cell Motil 31(1):45–57
Ryan TE, Brizendine JT, Backus D, McCully KK (2013) Electrically induced resistance training in individuals with motor complete spinal cord injury. Arch Phys Med Rehabil 94(11):2166–2173
Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM (2006) PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103(44):16260–16265
Scremin AM, Kurta L, Gentili A, Wiseman B, Perell K, Kunkel C, Scremin OU (1999) Increasing muscle mass in spinal cord injured persons with a functional electrical stimulation exercise program. Arch Phys Med Rehabil 80(12):1531–1536
Shah PK, Ye F, Liu M, Jayaraman A, Baligand C, Walter G, Vandenborne K (2014) In vivo (31)P NMR spectroscopy assessment of skeletal muscle bioenergetics after spinal cord contusion in rats. Eur J Appl Physiol 114(4):847–858
Singh K, Hood DA (2011) Effect of denervation-induced muscle disuse on mitochondrial protein import. Am J Physiol Cell Physiol 300(1):C138–C145
Stewart BG1, Tarnopolsky MA, Hicks AL, McCartney N, Mahoney DJ, Staron RS, Phillips SM. (2004) Treadmill training-induced adaptations in muscle phenotype in persons with incomplete spinal cord injury. Muscle Nerve. 30(1):61–68
Stolle S, Ciapaite J, Reijne AC, Talarovicova A, Wolters JC, Aguirre-Gamboa R, van der Vlies P, de Lange K, Neerincx PB, van der Vries G, Deelen P, Swertz MA, Li Y, Bischoff R, Permentier HP, Horvatovitch PL, Groen AK, van Dijk G, Reijngoud DJ, Bakker BM (2018) Running-wheel activity delays mitochondrial respiratory flux decline in aging mouse muscle via a post-transcriptional mechanism. Aging Cell. 17(1)
St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127(2):397–408
Talmadge RJ, Castro MJ, Apple DF Jr, Dudley GA (2002) Phenotypic adaptations in human muscle fibers 6 and 24 wk after spinal cord injury. J Appl Physiol (1985) 92(1):147–154
Tesch PA, von Walden F, Gustafsson T, Linnehan RM, Trappe TA (2008) Skeletal muscle proteolysis in response to short-term unloading in humans. J Appl Physiol (1985) 105(3):902–906
Tischler ME, Rosenberg S, Satarug S, Henriksen EJ, Kirby CR, Tome M, Chase P (1990) Different mechanisms of increased proteolysis in atrophy induced by denervation or unweighting of rat soleus muscle. Metabolism 39(7):756–763
Vainshtein A, Desjardins EM, Armani A, Sandri M, Hood DA (2015) PGC-1alpha modulates denervation-induced mitophagy in skeletal muscle. Skelet Muscle 5:9
Wade RC, Gorgey AS (2017) Anthropometric prediction of skeletal muscle cross-sectional area in persons with spinal cord injury. J Appl Physiol (1985) 122(5):1255–1261
Walsh B, Hooks RB, Hornyak JE, Koch LG, Britton SL, Hogan MC (2006) Enhanced mitochondrial sensitivity to creatine in rats bred for high aerobic capacity. J Appl Physiol (1985) 100(6):1765–1769
Wanga Y, Pessina JE (2013) Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr Opin Clin Nutr Metab Care 16(3):243–250
Weiss N, Andrianjafiniony T, Dupre-Aucouturier S, Pouvreau S, Desplanches D, Jacquemond V (2010) Altered myoplasmic Ca(2) handling in rat fast-twitch skeletal muscle fibres during disuse atrophy. Pflügers Arch 459(4):631–644
Whidden MA, Smuder AJ, Wu M, Hudson MB, Nelson WB, Powers SK (2010) Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J Appl Physiol (1985) 108(5):1376–1382
Wu H, Rothermel B, Kanatous S, Rosenberg P, Naya FJ, Shelton JM, Hutcheson KA, DiMaio JM, Olson EN, Bassel-Duby R, Williams RS (2001) Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J 20(22):6414–6423
Wu Y, Zhao J, Zhao W, Pan J, Bauman WA, Cardozo CP (2012) Nandrolone normalizes determinants of muscle mass and fiber type after spinal cord injury. J Neurotrauma 29(8):1663–1675
Wu Y, Collier L, Qin W, Creasey G, Bauman WA, Jarvis J, Cardozo C (2013) Electrical stimulation modulates Wnt signaling and regulates genes for the motor endplate and calcium binding in muscle of rats with spinal cord transection. BMC Neurosci 14:81
Yarar-Fisher C, Bickel CS, Kelly NA, Windham ST, McLain AB, Bamman MM (2014) Mechanosensitivity may be enhanced in skeletal muscles of spinal cord-injured versus able-bodied men. Muscle Nerve 50(4):599–601
Youle RJ, Karbowski M (2005) Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol 6(8):657–663
Yu T, Robotham JL, Yoon Y (2006) Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA 103(8):2653–2658
Yu T, Sheu SS, Robotham JL, Yoon Y (2008) Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res 79(2):341–351
Yu T, Jhun BS, Yoon Y (2011) High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxid Redox Signal 14(3):425–437
Zeman RJ, Zhao J, Zhang Y, Zhao W, Wen X, Wu Y, Pan J, Bauman WA, Cardozo C (2009) Differential skeletal muscle gene expression after upper or lower motor neuron transection. Pflugers Arch 458(3):525–535
Zhao J, Su Z, Qu C, Dong Y (2017) Effects of 12 Weeks Resistance Training on Serum Irisin in Older Male Adults. Front Physiol 8:171
Acknowledgements
The work is supported by the Department of Veteran Affairs, Veteran Health Administration, Rehabilitation Research and Development Service (B7867-W and B-2020-C) and DoD-CDRMP (W81XWH-14-SCIRP-CTA).
Author information
Authors and Affiliations
Contributions
ASG: provided funding support, developed the research hypothesis, submitted initial proposal, reviewed scientific evidence, helped writing and editing, and approved the final version. OW: reviewed scientific writing, summarized research finding, helped writing and editing, and approved the final version. LO’B: provided support for scientific writing, developed figures, helped editing and approved the final version. CC: provided critical feedback, assisted in scientific writing, helped editing, organizing and approved the final version. QC: provided support for scientific writing, developed figures, helped editing and approved the final version. EJL: provided critical feedback, assisted in scientific writing, helped editing, and approved the final version. ZAG: provided critical feedback, assisted in scientific writing, helped editing, organizing and approved the final version.
Corresponding author
Additional information
Communicated by Michael Lindinger.
Rights and permissions
About this article
Cite this article
Gorgey, A.S., Witt, O., O’Brien, L. et al. Mitochondrial health and muscle plasticity after spinal cord injury. Eur J Appl Physiol 119, 315–331 (2019). https://doi.org/10.1007/s00421-018-4039-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-018-4039-0