Abstract
Purpose
This study aimed to determine whether an increase in muscle shear modulus measured 30 min after eccentric exercise (30 min) reflects the magnitude of force deficit measured 48-h post-exercise (48 H).
Methods
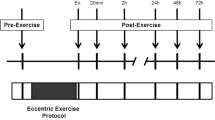
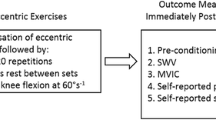
A total of 53 healthy participants were distributed in five groups. Four groups performed either repeated eccentric elbow flexions or knee extensions at either a low or high load. A fifth group performed repeated concentric elbow flexions (control load).
Results
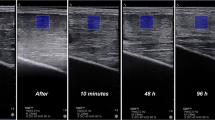
A significant decreased peak torque was found for elbow flexors and knee extensors 48 h after the eccentric exercises (all P values < 0.001). A significant increase in shear modulus was found at 30 min for the elbow flexors for low (+70.5 ± 44.3%, P < 0.001) and high load (+153.9 ± 192.4%, P < 0.001). Similarly, the shear modulus of knee extensors increased for low (+26.7 ± 19.1%, P < 0.001) and high load (+79.4 ± 67.1%, P < 0.001). The relative increase in shear modulus measured at 30 min was significantly correlated to the relative decrease in peak torque measured at 48 H for both elbow flexors (r = −0.80) and knee extensors (r = −0.82). A further analysis suggested that biceps brachii and rectus femoris were more affected by muscle damage than their synergists.
Conclusion
This study shows that an increase in muscle shear modulus measured 30 min after a damaging exercise reflects the decrease in peak torque measured at 48 H. Shear modulus may therefore, provide a useful tool for coaches and clinicians to non-invasively estimate the amount of muscle damage induced by a damaging exercise.





Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- BA:
-
Brachialis
- BB:
-
Biceps brachii
- MVC:
-
Maximal voluntary contraction
- RF:
-
Rectus femoris
- SSI:
-
Supersonic shear imaging
- VL:
-
Vastus lateralis
- VM:
-
Vastus medialis
References
Agten CA, Buck FM, Dyer L et al (2016) Delayed-onset muscle soreness: temporal assessment with quantitative MRI and shear-wave ultrasound elastography. AJR Am J Roentgenol. doi:10.2214/AJR.16.16617
Balnave CD, Allen DG (1995) Intracellular calcium and force in single mouse muscle fibres following repeated contractions with stretch. J Physiol 488:25
Balnave CD, Allen DG (1996) The effect of muscle length on intracellular calcium and force in single fibres from mouse skeletal muscle. J Physiol 492:705–713
Beaton LJ, Tarnopolsky MA, Phillips SM (2002) Variability in estimating eccentric contraction-induced muscle damage and inflammation in humans. Can J Appl Physiol 27:516–526
Bercoff J, Tanter M, Fink M (2004) Supersonic shear imaging: a new technique for soft tissue elasticity mapping. Ultrason Ferroelectr Freq Control IEEE Trans 51:396–409
Bouillard K, Hug F, Guével A, Nordez A (2012a) Shear elastic modulus can be used to estimate an index of individual muscle force during a submaximal isometric fatiguing contraction. J Appl Physiol (1985) 113:1353–1361. doi:10.1152/japplphysiol.00858.2012
Bouillard K, Nordez A, Hodges PW et al (2012b) Evidence of changes in load sharing during isometric elbow flexion with ramped torque. J Biomech 45:1424–1429. doi:10.1016/j.jbiomech.2012.02.020
Butterfield TA, Herzog W (2005) Quantification of muscle fiber strain during in vivo repetitive stretch-shortening cycles. J Appl Physiol 99:593–602. doi:10.1152/japplphysiol.01128.2004
Capetanaki Y, Bloch RJ, Kouloumenta A et al (2007) Muscle intermediate filaments and their links to membranes and membranous organelles. Exp Cell Res 313:2063–2076. doi:10.1016/j.yexcr.2007.03.033
Chapman DW, Simpson JA, Iscoe S, Robins T, Nosaka K (2013) Changes in serum fast and slow skeletal troponin I concentration following maximal eccentric contractions. J Sci Med Sport 16:82–85
Chen W, Ruell PA, Ghoddusi M et al (2007) Ultrastructural changes and sarcoplasmic reticulum Ca2+ regulation in red vastus muscle following eccentric exercise in the rat: effects of eccentric exercise on SR Ca2+ regulation. Exp Physiol 92:437–447. doi:10.1113/expphysiol.2006.036442
Cohen JH (1988) Statistical power for the behavioral sciences. REMEDICA Publishing, London
Davis J, Kaufman KR, Lieber RL (2003) Correlation between active and passive isometric force and intramuscular pressure in the isolated rabbit tibialis anterior muscle. J Biomech 36:505–512. doi:10.1016/S0021-9290(02)00430-X
Eby SF, Song P, Chen S et al (2013) Validation of shear wave elastography in skeletal muscle. J Biomech 46:2381–2387. doi:10.1016/j.jbiomech.2013.07.033
Eby SF, Cloud BA, Brandenburg JE et al (2015) Shear wave elastography of passive skeletal muscle stiffness: influences of sex and age throughout adulthood. Clin Biomech 30:22–27. doi:10.1016/j.clinbiomech.2014.11.011
Eby S, Zhao H, Song P et al (2016) Quantitative evaluation of passive muscle stiffness in chronic stroke. Am J Phys Med Rehabil 95:899–910. doi:10.1097/PHM.0000000000000516
Farahmand F, Sejiavongse W, Amis AA (1998) Quantitative study of the quadriceps muscles and trochlear groove geometry related to instability of the patellofemoral joint. J Orthop Res 16:136–143
Green MA, Sinkus R, Gandevia SC et al (2012) Measuring changes in muscle stiffness after eccentric exercise using elastography: measuring muscle stiffness changes after eccentric exercise using mre. NMR Biomed 25:852–858. doi:10.1002/nbm.1801
Guilhem G, Doguet V, Hauraix H et al (2016) Muscle force loss and soreness subsequent to maximal eccentric contractions depend on the amount of fascicle strain in vivo. Acta Physiol 217:152–163. doi:10.1111/apha.12654
Herzog W (2014) Mechanisms of enhanced force production in lengthening (eccentric) muscle contractions. J Appl Physiol 116:1407–1417. doi:10.1152/japplphysiol.00069.2013
Hoang PD, Herbert RD, Gandevia SC (2007) Effects of eccentric exercise on passive mechanical properties of human gastrocnemius in vivo. Med Sci Sports Exerc 39:849–857. doi:10.1249/MSS.0b013e318033499b
Hopkins WG (2000) Measures of reliability in sports medicine and science. Sports Med 30:1–15
Jamurtas AZ, Theocharis V, Tofas T et al (2005) Comparison between leg and arm eccentric exercises of the same relative intensity on indices of muscle damage. Eur J Appl Physiol 95:179–185. doi:10.1007/s00421-005-1345-0
Johnson MA, Sideri G, Weightman D, Appleton D (1973) A comparison of fibre size, fibre type constitution and spatial fibre type distribution in normal human muscle and in muscle from cases of spinal muscular atrophy and from other neuromuscular disorders. J Neurol Sci 20:345–361
Kawakami Y, Nakazawa K, Fujimoto T et al (1994) Specific tension of elbow flexor and extensor muscles based on magnetic resonance imaging. Eur J Appl Physiol 68:139–147
Kulig K, Powers CM, Shellock FG, Terk M (2001) The effects of eccentric velocity on activation of elbow flexors: evaluation by magnetic resonance imaging. Med Sci Sports Exerc 33:196–200
Lacourpaille L, Hug F, Bouillard K et al (2012) Supersonic shear imaging provides a reliable measurement of resting muscle shear elastic modulus. Physiol Meas 33:N19–28. doi:10.1088/0967-3334/33/3/N19
Lacourpaille L, Nordez A, Hug F (2013) Influence of stimulus intensity on electromechanical delay and its mechanisms. J Electromyogr Kinesiol Off J Int Soc Electrophysiol Kinesiol 23:51–55. doi:10.1016/j.jelekin.2012.06.010
Lacourpaille L, Nordez A, Hug F et al (2014) Time-course effect of exercise-induced muscle damage on localized muscle mechanical properties assessed using elastography. Acta Physiol 211:135–146. doi:10.1111/apha.12272
Lieber R, Thornell E, Friden J (1996) Muscle cytoskeletal disruption occurs within the first 15 min of cyclic eccentric contraction. J Appl Physiol 80:278–284
Opar MDA, Williams MD, Shield AJ (2012) Hamstring strain injuries. Sports Med 42:209–226
Paddon-Jones D, Keech A, Lonergan A, Abernethy P (2005) Differential expression of muscle damage in humans following acute fast and slow velocity eccentric exercise. J Sci Med Sport 8:255–263
Paulsen G, Crameri R, Benestad HB, Fjeld JG, Morkrid L, Hallen J, Raastad T (2010) Time course of leukocyte accumulation in human muscle after eccentric exercise. Med Sci Sports Exerc 42:75–85
Paulsen G, Mikkelsen UR, Raastad T, Peake JM (2012) Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise. Exerc Immunol Rev 18:42–97
Prior BM, Jayaraman RC, Reid RW et al (2001) Biarticular and monoarticular muscle activation and injury in human quadriceps muscle. Eur J Appl Physiol 85:185–190. doi:10.1007/s004210100434
Proske U, Morgan DL (2001) Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol 537:333–345
Raastad T, Owe SG, Paulsen G et al (2010) Changes in calpain activity, muscle structure, and function after eccentric exercise. Med Sci Sports Exerc 42:86–95. doi:10.1249/MSS.0b013e3181ac7afa
Salomoni S, Tucker K, Hug F et al (2016) Reduced maximal force during acute anterior knee pain is associated with deficits in voluntary muscle activation. PLoS One 11:e0161487. doi:10.1371/journal.pone.0161487
Stephenson D, Wendt I (1984) Length dependence of changes in sarcoplasmic calcium concentration and myofibrillar calcium sensitivity in striated muscle fibres. J Muscle Res Cell Motil 5:243–272
Takahashi H, Kuno S, Miyamoto T et al (1994) Changes in magnetic resonance images in human skeletal muscle after eccentric exercise. Eur J Appl Physiol 69:408–413
Warren GL, Lowe DA, Armstrong RB (1999) Measurement tools used in the study of eccentric contraction-induced injury. Sports Med 27:43–59. doi:10.2165/00007256-199927010-00004
Whitehead NP, Weerakkody NS, Gregory JE et al (2001) Changes in passive tension of muscle in humans and animals after eccentric exercise. J Physiol 533:593–604
Acknowledgements
This study was supported by grants from the French Ministry of Sports (Contract No. 15i19) and the Région Pays de la Loire (QUETE Project No. 2015-09035).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Additional information
Communicated by Olivier Seynnes.
Rights and permissions
About this article
Cite this article
Lacourpaille, L., Nordez, A., Hug, F. et al. Early detection of exercise-induced muscle damage using elastography. Eur J Appl Physiol 117, 2047–2056 (2017). https://doi.org/10.1007/s00421-017-3695-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3695-9