Abstract
Background
Investigations of the effect of beverages containing carbohydrates, only, on the sodium and fluid balance during intermittent exercise of high intensity are rare. Therefore, we compared the effects of water and carbohydrate supplementation on plasma, blood volume, and electrolyte shifts during intermittent exercise.
Methods
Ten male subjects performed an intermittent exercise test twice. In one trial, tap water (4 ml/kg/15 min) was consumed (Plac trial). In the other trial, the same amount of water supplemented with maltodextrin to achieve a 9.1 % carbohydrate solution (CHO trial) was ingested. Training schedule: warm-up at 50 % for 15 min. Afterwards, power changed between 100 % of the maximum power from a previous incremental test minus 10 and 10 W for each 30 s. Venous blood was sampled to measure electrolytes, osmolality, [protein], hct, [Lactate], [glucose], [Hb] and catecholamines. Hydration status was evaluated by BIA before and after exercise.
Results
After beverage ingestion [glucose] was significantly higher in CHO until the end of the trial. Starting with similar resting values, osmolality increased significantly more during CHO (p = 0.002). PV decreased by 5 % under both conditions, but recovered partly during exercise under Plac (p = 0.002). [Na+] and [Cl−] decreased with Plac during exercise (both p < 0.001) but remained constant during exercise with CHO.
Conclusions
Sole carbohydrate supplementation seems to stabilise plasma [Na+]. This cannot be explained simply by a cotransport of glucose and [Na+], because that should lead to a recovery of the blood and plasma volume under CHO. In contrast, this was found during exercise with Plac.
Similar content being viewed by others
Introduction
Lots of studies have investigated the effects of carbohydrate (CHO) substitution on water and electrolyte balance during continuous endurance training (CET) (e.g., Criswell et al. 1992; Davis et al. 1988; Fritzsche et al. 2000). To the best of our knowledge, this is not the case for intermittent training with high intensity.
It is known that CHO consumption during CET delays the onset of fatigue (Jeukendrup and McLaughlin 2011) and that maintaining balanced water and electrolyte homeostasis is essential in delaying exhaustion or even more severe conditions like exercise-associated hyponatremia (Lamb and Brodowicz 1986; Maughan and Meyer 2013; Murray et al. 2003). A recent study showed a high incidence of hyponatremia during a training camp with prolonged high-volume training in rowers, which was partially attributed to overdrinking (Mayer et al. 2015). This study shows that the amount of fluid substitution and the composition of sports drinks during exercise of varying intensities are still an actual problem.
The fluid and electrolyte homeostasis and fluid substitution for CET have been extensively studied in the past. Criswell et al. found a better maintenance of plasma volume (PV) when consuming a 7.1 % glucose-electrolyte drink during a CET of approx. 65 % VO2peak compared with a water placebo. [Na+] and [K+] did not differ between the trials in this study (Criswell et al. 1992, 1991). Similar results were shown comparing water to water-CHO solution (9.1 %) during cycling at 60 % VO2peak to exhaustion (Maassen et al. 1995). Davis et al. found no differences in PV when comparing 6 and 2.5 % CHO-electrolyte drinks to water during a 2-h continuous endurance exercise followed by another 30 min bout of more intense work, but did not measure any electrolytes (Davis et al. 1988).
However, the transferability between CET and training with high volumes of high intensities is questionable. During short intervals of high-intensity exercises, filtration pressures inside the muscle may differ from steady-state exercises. Furthermore, the muscle fibre recruitment pattern differs (Gollnick et al. 1974). Some studies show that more type 2 muscle fibres are used during high-intensity exercise and during intermittent exercise than during CET (Essen 1978a, b; Gollnick et al. 1974). Type 2 fibres have different metabolic and electrophysiologic properties. It was shown by Ruff that the number of Na+ channels in type 2 fibres is larger than in type 1 (Ruff 1996). The different amounts of membrane channels may lead to different shifts of [Na+] and [K+] between extracellular space and muscle (Clausen 2003) affecting the membrane potential (Cairns and Lindinger 2008). Stable concentrations of electrolytes, however, are the basis for preventing decreases in the compound action potential, and thus improve exercise performance (Bangsbo et al. 1996). Because of the increased anaerobic metabolism and the enhanced recruitment of type II fibres during exercise with high intensities, the intracellular osmolality will increase due to the intracellular accumulation of lactate and the products of the CRP breakdown (Maassen et al. 1998; Sjogaard and Saltin 1982) with a corresponding decrease in the extracellular space (Maughan and Meyer 2013). The resulting water shift influences the extracellular and the intracellular electrolyte concentrations and the compound action potential (Shushakov et al. 2007).
CHO supplementation during high-intensity training (HIT) or high-intensity interval training (HIIT) was investigated with various training protocols in different studies in the past. In these studies, different types of exercises like running and cycling as well as different intensities and durations were investigated (e.g., Murray et al. 1989; de Sousa et al. 2007; Nicholas et al. 1999; Nassis et al. 1998). Hence, these works are difficult to compare, because different muscle groups, masses and fibres are active during the different types of exercise. This can, for example, result in a different glucose disposal inside the body (Tsintzas et al. 2003). In addition, there are even fewer studies in which exercise lasted until exhaustion. An intermittent cycling exercise showed no differences between CHO and water consumption for the percentage change of plasma volume (PV) sodium and potassium (Murray et al. 1987). However, this exercise was performed in the heat and consisted of several 15 min periods of steady-state cycling (50 and 65 % VO2max) followed by only two performance rides of high intensity.
To the best of our knowledge, there are no studies investigating high volumes of high-intensity training lasting until exhaustion that focused on the changes in the water and electrolyte balance under CHO or water supplementation. That is why a “high-intensity high-volume training” (HIHVT) protocol was introduced by our laboratory (Maassen and Vardaxoglou 2006) and used in a previous study (Maassen et al. 2007) in which training and drinking protocols were similar to the present study and to the CET study of Maassen et al. (1995). In this investigation, the PV was significantly better maintained in the CHO group. This agrees with the findings mentioned above (Criswell et al. 1992; Maassen et al. 1995). However, there was a tendency for [Na+] to decrease towards hyponatremia in the placebo group during exercise (75 min cycling exercise). It remained unclear whether the observed differences would reach the level of significance later in exercise, since the subjects did not have to perform until exhaustion. Since this study was not a crossover study, the difference in [Na+] might have been due to individual differences among the participants. That is why the present study is designed as a crossover study with an HIHVT protocol lasting until exhaustion. The purpose of this study is to illustrate the water balance during an HIHVT under substitution of either water or water plus CHO. We hypothesise that CHO consumption stabilises the [Na+] and the PV and, thus, leads to a better exercise performance.
Methods
Subjects
Ten healthy male subjects (26.18 ± 3.19 years; 185.91 ± 5.66 cm; 82.32 ± 7.81 kg; 4.3 ± 0.51 W/kg) gave written informed consent to participate in this study, which was approved by the Ethics Committee of the Hannover Medical School. Five were recreational endurance trained athletes (4.67 ± 0.41 W/kg; 81.28 ± 6.64 kg) and five non-endurance trained but physically active subjects (3.91 ± 0.25 W/kg; 86.22 kg ± 9.58 kg).
Pretest
All participants performed a continuous incremental test on a cycle ergometer (Lode Excalibur Sport, Groningen, the Netherlands) to determine their maximum Power (P max), which was used as the basis for the HIHVT. After the incremental test, we used the CO rebreathing method (Schmidt et al. 1988; Burge and Skinner 1995; Thomsen et al. 1991) to determine the hb mass.
Experimental design
The experiment was designed as a single-blind crossover study. Each subject had to perform two HIHVT sessions on a cycle ergometer in which either a CHO solution or a water placebo was consumed (CHO trial vs Plac trial). Subjects were instructed not to exercise and to keep a nutrition diary 2 days prior to the HIHVT and to eat similar food before the second. Five subjects started with the CHO trial, and the other five with the Plac trial. The trials were separated by at least 5 days to avoid a training effect. Power (P) alternated during exercise from 100 % of the maximal power (P max) reached in the incremental test minus 10 to 10 W every 30 s until total exhaustion (mean P = 50 % P max). Subjects were instructed to maintain a constant pedalling rate of 80–90 revolutions per minute (rpm).
Schedule
(Measuring times are given in brackets.) On the day of the trial, all participants had the same breakfast consisting of one roll with jam at 07:00 a.m. Subjects reported to the laboratory at 8.00 a.m. Upon arriving, the participants voided their bladders, and their body weight was measured. Afterwards, the subjects lay down for 20 min before the first of four bio impedance analyses (BIA) (Nutriguard M, Data Input GmbH) was performed. The BIA was followed by the first blood sampling for pretrial values of [hb]v, hct, [prot]v and electrolytes in plasma (0 min). Subjects then mounted the cycle ergometer and were attached to an open-circuit spirometric system (Metalyzer 3B, Cortex Biophysik, Germany) to measure the ventilation (VE) and respiratory exchange ratio (RER). Heart rate was recorded by a Vantage NV™ heart rate monitor (Polar, Suomi). Time recording started after the attachment of all devices.
For the first 10 min, the subjects rested on the cycle, while the second blood sample was taken (5 min). After 10 min, they dismounted, the urine volume and weight were measured, and the first drink was ingested. After 20 min, the subjects mounted the ergometer again, and another blood sample was taken at 25 min. Subsequently, the warm-up (WA) started, consisting of 2 min cycling at 10 W and 15 min at 50 % P max. Another blood sample was drawn at the end of the warm-up (42 min). The HIHVT started at minute 42 and went on until exhaustion. Blood samples were drawn every 15 min (57–102 min). Exhaustion was defined as the inability of the subjects to maintain cycling at 80 revolutions per minute, or the feeling of subjective exhaustion. After terminating the HIHVT, another blood sample was drawn (113 min). This was followed by a 10-min cool-down period of 50 % Pmax (CD1) and another blood sampling at the end of this (125 min). The participants then dismounted, gave the last urine sample, and were weighed. About 20 min after terminating the HIHVT, blood was sampled again (CD 2, 138 min). Afterwards, the test subjects lay down to recover for about 40 min (LD). The trial ended 60 min after the HIHVT with the last blood sampling and BIA (173 min).
Spirometry
The spirometric measurements were taken during the warm-up and during the first 7 min of each interval period.
Drinks
Six ml/kg/bw of the beverages were ingested before the warm-up between minutes 12 and 20, 4 ml/kg/bw during the warm-up between minutes 22 and 40, and then 4 ml/kg/bw every 15 min during the HIHVT after removal of the spirometry mask. Hence, the drinking time was 8 min. For the CHO trial, the same amount of water as in the Plac trial was added with maltodextrin (Kristallpur®, STOCKMEYER FOOD) to achieve a 9.1 weight per cent CHO solution. Both drinks were supplemented with a small amount of lemon concentrate to achieve an equal taste and were kept at ambient temperature.
Blood samples
An 18-gauge catheter was placed in the cubital vein of the right forearm and kept patent during the trial by periodical injections of 2 ml physiological saline (NaCl 0.9 %, Braun). Ten millilitres of blood was sampled in a tube supplemented with 20 µl heparin (N2500, HOFMANN LA ROCHE, Melsungen). Six millilitres of these samples were used for immediate determination of electrolytes in venous plasma ([Na+]v, [K+]v, [Cl−]v), acid base status (ABL 505, Radiometer Copenhagen), [hb]v (OSM3 Hemoximeter, Radiometer Copenhagen), [hct]v (Na-heparinized Microtubes, B. BRAUN, Melsungen Zentrifuge), glucose [glu]v (Biosen 5130, EKF, Barleben, Germany) and lactate [lac]v (Biosen 5030, EKF). The remaining blood was centrifuged at 4 °C at 11,000 rpm. (3–18 K, SIGMA, Osterode), and the plasma used for the determination of plasma proteins [prot]v (Biuret Method) and osmolality in plasma [osm]v (Osmometer, Roebling). An additional sample tube of 8 ml was taken (except for at 72 and 87 min to reduce the amount of taken blood) and put in two EDTA tubes (S-MONOVETTE, Sarstedt). These samples were centrifuged at 4 °C at 5500 rpm, and the plasma stored at −70 °C for later determination of plasma noradrenaline [NA]v, adrenaline [A]v and insulin [Insulin]v with ELISA (DRG Instruments GmbH). Parallel to each venous blood sample, 20 µl of capillary blood was taken from the subjects’ hyperaemized earlobe for measurement of [glu]cap (Biosen 510, EKF) and [lac]cap (Biosen 5030, EKF).
Changes in PV were determined using the formula: BVA = BVB × (HbB/HbA); CVA = BVA(HctA); PVA = BVA − CVA; ∆PV % = (PVA − PVB)/PV)B; (A = after exercise, B = before exercise). (Dill and Costill 1974) The hb mass from the pretest was used to calculate the BVB. The baseline for the relative changes in PV was established at 25 min, because the exercise started immediately afterwards. In addition, the subjects had already spent at least 45 min in an upright position, so that we could exclude the influence of changes in body position on the PV.
Blood pressure was measured parallel to each blood sampling via a blood pressure cuff (Riva Rocchi) on the left upper arm during the 10-W phase between the intervals.
Urine volume was measured before mounting the cycle, before the first beverage was consumed and after exercise termination.
A tetrapolar multi-frequency BIA (Nutriguard M, Data Input GmbH; 5, 50, 100 Hz) was performed four times: lying before exercising (B1), sitting on the ergometer before exercising (B2), sitting just after the cool-down (B3) and lying 60 min after exercising (B4). The placement of the electrodes was standardised and marked on the participants’ wrists and ankles.
Ambient temperature
Trials were performed indoors. The temperature was continuously measured and kept at 22 °C via air conditioning.
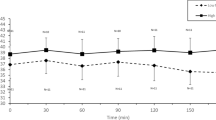
In another investigation from our laboratory, eight male subjects different from the present study performed a similar HIHVT protocol to the original study without supplementation of fluids. The subjects exercised on the same ergometer and the same techniques, and devices were used. Blood samples were drawn at rest, after the warm-up, during the HIHVT and after the cool-down. Only the [Na+]v was included for the present study (Fig. 1).
[Na+] in plasma (mmol l−1). Significant differences between CHO and Plac trials from 57 min (p = 0.023) until 173 min (p < 0.001). Interaction between trial and time (p < 0.001) in CHO and Plac trial. No interaction between trial and time in no-fluid. No fluid values based on data from additional investigation with different subjects
Statistical analysis
All data were analysed using a two-way repeated ANOVA to determine the level of significance. A Holm-Šídák post hoc test was used to locate significant differences. Significance level was set at p < 0.05. Values are presented as means ± standard deviations (sd) based on ten subjects (n = 10).
Results
No significant differences in the mean training duration could be detected between the Plac trial and the CHO trial (68:25 ± 18:55 vs 66:47 ± 19:53 min). The mean amount of consumed CHO was 141.88 ± 29.94 g. Mean water ingestion was 1559.1 ± 328.98 ml. The mean osmolality of the CHO drinks was 99.3 ± 1.53 mosmol/kg H2O. No differences in body weight were detected over time or between the trials. Mean urine volumes before and after the exercise were also not significantly different between the CHO and the Plac trial at any time. Total loss of urine was 228.82 ± 201.95 ml in the Plac trial and 146.05 ± 71.52 ml in the CHO trial. Heart rates were not significantly different between the trials. However, there was a trend to lower heart rates in Plac trial at the beginning of the exercise, which vanished at the end of exercise.
Glucose
Figure 2 starting with similar concentrations at rest (5 min) [glu]cap increased after beverage ingestion during the CHO trial, but not in the Plac trial (25 min). With the beginning of the warm-up [glu]cap decreased on both occasions. In the Plac trial, concentrations dropped below the resting values and remained at this level even after exercise termination. In the CHO trial, the concentration increased above initial values during the course of exercise with a peak after exercise termination (138 min). Interestingly, while the concentration of [glu]cap was significantly higher in the CHO trial shortly after beverage ingestion (25 min, p < 0.001), the difference between the trials did not change significantly during exercise (125 min, p < 0.001). The time course of [glu]cap in both trials seems to be equal on two different levels during exercise. The [glu]ven showed the same pattern during the whole trial (not shown).
Capillary glucose (mmol l−1). Significant differences between trials from 25 until 173 min (p < 0.001). Interaction between trial and time (p < 0.001). Upper Y axis: R rest, Drink supplementation of the first drink, WA warm-up, Intervals, CD1 + 2 = cool-down 1 + 2, LD lay down, see “Methods”
Electrolytes and osmolality
In the CHO trial, [osm]v (Fig. 3) continuously increased from resting values (5 min) until exercise termination (113 min, p = 0.001), and afterwards decreased to initial values. In the Plac trial, [osm]v remained constant until the end of exercise (113 min), and decreased below resting values afterwards (173 min). The differences between the trials were significant from the first bout of intervals (57 min, p = 0.028) until the end (173 min, p < 0.001). The course of [Na+]v was similar to that of [osm]v. [Na+]v increased from resting values (5 min) after the start of the HIHVT (52 min, p < 0.001) and remained constant until exercise termination (113 min) in the CHO trial. Afterwards, it decreased below initial values (173 min). During the Plac trial, [Na+]v decreased from initial values (5 min) during the course of exercise (125 min) and even further afterwards (173 min). The difference between the trials was significant after the first bout of intervals until the end of the trial (173 min, p < 0.001). The pretrial values for [Na+]v (0 min) were 137.7 ± 1.62 mmol/l in the CHO trial and 138.1 ± 1.29 mmol/l in the Plac trial, and are not shown in Fig. 4 for reasons of clarity. [Na+]v during exercise without fluid supplementation increased slightly. The time course resembles that of the CHO trial on a higher level.
The course of [Cl−]v (Table 1) parallels that of [Na+]v. There were differences between the groups during exercise (97 min, p = 0.02) which remained until the end (173 min, p = 0.002). There were no differences in [K+]v (Table 1) between the Plac trial and the CHO trial. In both cases, [K+]v increased during exercise and then fell back to initial values (Table 1). However, the rate of the decrease in [K+]v from post-exercise (125 min) to 60 min post-exercise (173 min) was significantly faster in the CHO trial than in the Plac trial.
Plasma volume
The initial absolute (Fig. 4) PV at baseline (25 min) was 3615 ± 649 ml in the Plac trial and 3614 ± 536 ml in the CHO trial. The small differences between the trials in [hb]v and hct shown in Table 1 were not statistically significant. However, [hb]v, hct and [prot]v increased during the exercise and returned to baseline at the end in both trials. In accordance with that, the relative changes in the PV were also significant during the course of the training. In both trials, the PV decreased during the exercise and recovered completely at the end of the trial while resting. However, the PV recovered faster in the Plac trial. At the end of the exercise, the PV was 80 ml higher in the Plac trial (113 min, p = 0.025) and afterwards 110 ml higher (125 min, p = 0.01) than in the CHO trial. At the same time, there were no significant differences in [prot]v.
Lactate
The [lac]cap increased significantly (Table 1) during the HIHVT in both trials (p < 0.001). The peak concentrations during the Plac trial were higher than during the CHO trial from the first bout of intervals (52 min, p = 0.032) until the end of the HIHVT (113 min, p = 0.007).
Insulin
Starting with similar baseline values (Table 1), [Insulin]v increased in both trials after drinking the first beverage, but significantly more strongly in the CHO trial (5 min, p = 0.012). With the beginning of the warm-up, the concentration decreased in both cases but remained on a higher level in the CHO trial. After the first bout of intervals, the difference between the trials remained significant for the whole trial (57 min, p = 0.013). After the cool-down period, [Insulin]v increased under both conditions, but to a much larger extent with CHO feeding than with water (138 min, p < 0.001).
Catecholamines and blood pressure
Concentrations of [NA]v and [A]v and the blood pressure (Table 1) increased during exercise (p < 0.001). [NA]v decreased to initial values after the exercise, while [A]v decreased even below initial values in the CHO trial. However, only in [A]v did significant, differences between the trials occur. From the last bout of intervals (102 min, p = 0.007) until the end of the trial (173 min, p < 0.001), [A]v was significantly higher in the Plac trial.
Bio impedance analysis
The ECW, ICW and TBW increased significantly (Table 2) during the exercise (B2–B3) in both trials. From B1 to B4, the increase was also significant except for the ECW in the CHO trial. However, there were no statistically significant differences between the Plac trial and the CHO trial at any time in TBW, ECW or ICW.
Discussion
The purpose of this study was to investigate the time course of the [Na+] and the changes in PV during HIHVT when consuming either CHO or water. To observe the sole effect of CHO, we chose a high dose of maltodextrin without adding electrolytes to achieve a high carbohydrate load with low osmolality. The main finding of this study is that the PV declined similarly at the beginning of both trials during exercise but partially recovered in the Plac trial at the end of exercise but not in the CHO trial. [Na+]v decreased with water supplementation but was stabilised comparably to exercise without supplementation by ingesting water plus CHO.
Methodical considerations
Keeping a nutrition diary and following the training advices guaranteed an equal food and fluid intake and similar physical activity 2 days before the trials. This led to similar data for body weight, RER, [glu]cap and [lac]cap at rest and before the start of supplementation, showing that there were no differences in the nutrition status at the beginning of the experiment (Maassen and Busse 1989). The similarity of TBW, [hct]v, urine volume, body weight, PV, and BV under both conditions before the experiments indicates a comparable hydration status. The loss of fluids displayed by the urine output, changes in body weight and TBW and the VE to estimate the fluid loss by breathing were not different either. There is evidence from CET studies that consumption of either CHO or water does not lead to any different rates of water and electrolyte loss via sweating (Criswell et al. 1992; Davis et al. 1988; Sanders et al. 2001).
BIA measurements are accurate in a euhydrated, rested state (Kyle et al. 2004; O’Brien et al. 2002). In the present study, this applies to B1 and to B4. However, alterations in electrolyte balance and skin temperature can influence the BIA measurements independently from actual fluid changes (O’Brien et al. 2002). That means that B2 and B3, which were performed shortly after exercise, are not suitable for quantifying the exact amount of water shifts between the compartments. However, since ambient temperature, mean exercise duration, mean P, change of body weight and consumed water were the same during both trials, we conclude that all the collected BIA data are qualitatively valid for interpreting differences between the trials. The results show that there seems to be no difference in TBW, ECW or ICW between the two trials. Our results are supported by findings of Baker et al. showing that changes in body weight correlate accurately with the changes in body water during exercise (Baker et al. 2009).
The relative changes in the PV were calculated using the formula given by Dill and Costill (1974). The baseline for the relative changes in PV was established at 25 min, because the exercise, our main interest, started immediately afterwards and the subjects had already spent at least 45 min in an upright position. The absolute PV was calculated with a total hb mass, which was measured after the incremental test and [hb]v and hct measured at the beginning of the HIHVT. According to Schmidt and Prommer (Schmidt 2005), the hb mass remains constant as long as the physical condition does not change. This applies to our study design, because each subject’s individual training status was similar before the trials and the experiments were performed in an alternating sequence.
The subject sample consisted of five recreational endurance athletes and five physically active subjects. Although there was a significant difference in the relative power between the groups (4.67 vs 3.91 W/kg, p = 0.0085), the difference was small. We can expect no large difference in muscle fibre phenotypes, since the total range of relative power between untrained and highly trained endurance athletes reaches from 3 to 7 W/kg, so both groups can be considered moderately trained.
Our main findings are somewhat unexpected and contrary to previous works (Criswell et al. 1992; Maassen et al. 1995). In the two mentioned studies, the exercise intensity matched the mean intensity in our study, but no significant changes in [Na+]v were found, and the PV was significantly better maintained when CHO was consumed during exercise. However, Davis et al. found no changes in PV when comparing two CHO-electrolyte drinks to water ingestion during a CET (Davis et al. 1988).
Criswell et al. explained the smaller decrease in the PV when consuming CHO during CET by the cotransport of glucose and sodium by the sodium-dependent glucose transporter (SGLT1) located in the apical brush border membrane of the enterocytes (Criswell et al. 1991) and a subsequent water resorption. Since we observed a significant increase in [Na+] v, [Cl−]v and [glu]cap, which accounts very accurately for the increased [osm]v, we would have expected the same in the present study. However, this was not the case. At the end of the HIHVT, the PV was even significantly larger in the Plac trial. Since the hct, [hb]v, [prot]v and ECW were not different between the trials, a dilution effect is improbable. It is notable that the decrease in [Na+]v occurred shortly after the beginning of the exercise only in the Plac trial, while the PV declined to the same extent in both trials. The difference in [Na+]v and [osm]v remained throughout the whole exercise, while the differences in PV occurred only at the end of the training. This leads to the conclusion that the developments of [Na+]v, [osm]v and PV are largely independent of each other. In the additional experiment without water supplementation, [Na+]v increased slightly during the exercise. This shows that supplementation of water alone during a training of high intensity leads to a significant decrease in [Na]v, and agrees with previous studies (Anastasiou et al. 2009; Vrijens and Rehrer 1999). However, in these works, water supplementation was compared with beverages containing electrolytes and carbohydrates. Our data show that CHO supplementation without added electrolytes results in an increase in [Na+]v comparable to exercise without any supplements.
Since fluid intake and fluid loss are equal between the trials, the reasons for the independent development of PV and electrolytes should be found in the gastrointestinal absorption, the capillary filtration, and a different distribution among the body compartments.
Gastrointestinal absorption
The intestinal fluid absorption during exercise depends on several factors, such as the beverage osmolality, absorbable substrates, type and amount of CHO and the rate of gastric emptying (Shi and Passe 2010; Shi et al. 1995; Shi and Gisolfi 1998; Jeukendrup and McLaughlin 2011). To the best of our knowledge, no differences in gastric emptying during exercise have yet been observed between CHO beverages of low osmolality and water (Leiper et al. 2005; Gisolfi et al. 1998). In the present study, both drinks were hypoosmolar. The increase in [Na+]v and [gluc]cap in the CHO trial could indicate an enhanced absorption via cotransport in the enterocytes. This should have promoted fluid absorption (Shi et al. 1995) and, consequently, a larger PV, which was not the case. A possible explanation is that maltodextrin hydrolysis in the intestine has at some point exceeded the rate of glucose absorption and thus increased the intraluminal glucose concentration and osmolality. The further increase in the blood glucose concentration after the training when no new drinks were consumed could indicate that a great amount of glucose was still present in the intestine at this point of the trial. Jeukendrup and McLaughlin reported a max. CHO oxidation rate of 1 g/min for single saccharides during exercise. They suggested that a limitation in the capacity of the SGLT1 is responsible for this ceiling (Jeukendrup and McLaughlin 2011). In the present study, the participants consumed solely maltodextrin at a rate of 1.62 g/min. An osmotic effect of hydrolyzed glucose could appear late in exercise when big amounts of maltodextrin have already been consumed. When Shi et al. infused an 8 % glucose solution into the duodenojejunum of resting subjects the group also detected a decrease in the plasma volume (Shi et al. 1995). However, in the present study, no rates of intestinal fluid absorption were measured. If an increased intraluminal osmolality had caused a reduced water absorption in the CHO trial, the PV in the Plac trial should have increased during the exercise, because the same amount of water without glucose was supplemented. In fact, this was the case at the end of the exercise. Nevertheless, it is questionable whether this increase in the PV is due to a larger water reabsorption, because [prot]v, [hb] and hct behave similarly under both conditions.
Capillary filtration
Since [prot]v was not different between the trials, we assume equal colloid osmotic pressures in both cases. In addition, in [NA]v, no differences were detected, but [A]v reached significantly higher concentrations in the Plac trial. This is consistent with several previous studies that showed that glucose ingestion reduced [A]v before and during exercise (De Bock et al. 2007; Nicholas et al. 1999; Petersen et al. 2003). However, the higher [A]v was not paralleled by significant differences in the mean systolic or diastolic blood pressure. This could be due to the fact that the effect of vasoconstrictors in the microcirculation of working muscle can be compensated for by local vasodilating factors, such as, for example, NO, CO2, lactate or K+ (Hellsten et al. 2012; Clifford and Hellsten 2004). Moreover, the effect of catecholamines on the filtration pressure varies in different parts of the body, such as exercising and non-exercising muscles and liver (Nielsen et al. 2007). That is why we suggest that the increased [A]v in the CHO trial did not cause significant differences in filtration and fluid shifts between the trials.
Glucose cannot freely cross from intravascular to interstitial space because of the reflection coefficient at the capillary wall (Rippe 2008; Michel and Curry, 1999). Hence, glucose could contribute to an enhanced PV by creating an osmotic gradient across the capillary wall. Since the PV in the CHO trial is not larger than in the Plac trial, we assume that the effect of the reflection coefficient is negligible.
Fluid distribution
A different substrate utilisation can cause distinct intra- and extracellular substrate concentrations and thus lead to different fluxes of water across the cellular membrane (Maassen et al. 1998; Sjogaard and Saltin 1982). In the present study, [lac]cap was significantly higher in the Plac trial than in the CHO trial. However, the differences in concentrations were very small (Table 1) and are partially counterbalanced by a decrease in [HCO3 −] (data not shown). Hence, the differences in both of these concentrations are not relevant for [osm]v, since the differences in osmolality can largely be attributed to [Na+]v, [Cl−]v and [glu]cap.
However, the CHO metabolism could also play an important role. The intracellular glucose uptake rises in proportion to the extracellular glucose concentration (Rose and Richter 2005). Thus, high [glu]cap can result in spared intracellular glycogen stores (De Bock et al. 2007). Glycogen utilisation during high-intensity exercise, while consuming carbohydrate drinks is reduced by 22 % compared to a placebo (Nicholas et al. 1999). In the present study, the RER was significantly lower in the Plac trial at the end of the exercise with unchanged VE, which indicates that more fatty acid oxidation occurs at this point in the Plac trial. This could account for the more exhausted glycogen stores at the end of exercise in the Plac trial than in the CHO trial and would support the findings of the group around Nicholas. One g glycogen can bind approximately 2.7 g water inside the muscle (Koulmann et al. 2000). Essen et al. found a decrease in glycogen concentration in the working muscle of 8.5 g kg−1 during 60 min of intermittent training of a mean intensity comparable to ours (Essen et al. 1977). The mass of active muscles during ergometer cycling is approximately 10 kg (Nielsen et al. 1984). Given the mean training duration in our study, this results in a release of 259 ml of water during the H2O trial (8.5 × 10 × 2.7 × 1.13) and 202 ml during the CHO trial (22 % of 259 ml) from glycogen stores. However, in a previous study, hyperglycaemic athletes had no altered muscle glycogen utilisation when being hyperglycaemic during intense cycling exercise (Coyle et al. 1991). Moreover, water “bound” by glycogen would still be influenced by other osmotic gradients, as it is already a part of the TBW, so the effect would most likely be small (Murray et al. 2003). Therefore, in summary, the sparing of glycogen stores in the CHO trial might contribute to the observed differences in the PV at the end of the exercise, though it is unlikely to be the main reason.
A faster recovery of the PV under substitution of water compared to CHO, as in the Plac trial at the end of exercise in the present study, has not been observed in other investigations. Interestingly, we found no differences in the concentration of proteins between the trials at the same time, although the increase in PV with Plac should lead to a decrease in [prot]v. The decrease in the PV was about 5 % from baseline until termination of exercise in the CHO trial with a corresponding increase in [prot]v of about 5 %. In the Plac trial, the [prot]v also increased 5 % in the same time, but the PV increases at the end of the exercise, which is unexpected and can hardly be explained by a sudden protein resorption into the vascular bed. Since we used the formula given by Dill and Costill (1974), a reliable determination of the hct is essential for correct calculations of the PV. During rest, the hct in big, central vessels is significantly larger than in small vessels and capillaries. During exercise, the hct in capillaries and small vessels can increase significantly due to an enhanced perfusion (Klitzman and Duling 1979; Poole 2014; Fricke 1965) and thus influence the measured hct in big vessels compared to rest. Oral glucose intake with a consequently increased [Insulin]v can impair the myogenic vasoconstriction response to increased transmural pressure without changes in the mean artery blood pressure (Lott et al. 2007). In the present study, [glu]cap and [Insulin]v were significantly larger in the CHO trial. Thus, it is possible that the difference in the hct in the cubital vein is rather an effect of enhanced capillary perfusion due to exercise or an altered myogenic response with glucose than of an actual expansion of the PV. A small change in hct can cause a significant difference in the calculated PV as shown in the following example using the Dill and Costill method:
BVA = BVB × (HbB/HbA); CVA = BVA(HctA); PVA = BVA − CVA; ∆PV % = (PVA − PVB)/PV)B; (CV = cell volume, BV = blood volume, PV = plasma volume and A = after exercise and B = before exercise = baseline).
If we assume BVB = 5.5 l; HbB = 15 g/dl; Hct A = 0.45, and a constant MCHC of 35 g/dl, then HbA = 15.75; BVA = 5.24; CVA = 2.358 and PV A = 2.882.
If Hct B = 0.46 with the same baseline values and MCHC, then HBA = 16.1; BVA = 5.12; CVA = 2.357 and PV A = 2.763 l (the relative changes of PV are significant, respectively).
The calculated difference of about 119 ml is caused by a change in hct of 1 %. It has the same order of magnitude as the observed PV differences between the CHO trial and the Plac trial in the present study and also agrees with a previous study reporting a difference between measured and calculated PV of 111 ml (Van Beaumont et al. 1972). All considered that this makes it highly probable that the higher calculated PV at the end of the exercise in the Plac trial is caused by an overestimation of the real PV.
Electrolyte distribution
Since there is no PV expansion during the CHO trial, the reabsorption from the intestine cannot be the only reason for the higher [Na+]v in the CHO trial. There is the recent literature (Titze 2015) reporting about storage sites for [Na+] under the skin and inside the muscle (Titze 2014), which do not promote fluid retention. As early as 1967, Cope stated that large parts of intramuscular Na+ seem to be complexed with actin and myosin (Cope 1967). Titze suggested that there is not necessarily equilibrium between sodium concentration in body compartments and the amount of retained fluids (Titze 2014). Noakes et al. also partly attributed hyponatremia during endurance events to osmotical inactivation of sodium or failure to mobilise osmotical inactivated sodium (Noakes et al. 2005). Whether these sodium stores have any influence on short-term adaptations during HIHVT is a matter of speculation, but glucose intake might trigger a release of sodium from these complexed stores independently of fluid shifts.
Moreover, during intense exercise, the muscle has to create a high rate of action potentials with subsequent diffusion of Na+ into the muscle via Na+ channels and outward diffusion of K+ (Green et al. 2007). Type 2 muscle fibres contain more sodium channels than type 1 fibres (Ruff 1996), which will enhance these inward Na+ shifts during action potentials during HIHVT compared with CET. It has been shown that during intense exercise, there is an intracellular uptake of Na+ (Lindinger et al. 1992; Sjogaard 1990a; Sjogaard et al. 1985) and, thus, a decreased extracellular Na+ content without CHO feeding. The Na+/K+ pump actively counteracts these ion shifts by transporting sodium in, and potassium out, of the muscle.
Glucose supplementation can prevent deterioration of electrical properties in the muscle fibre membrane (Karelis et al. 2002) and protect the muscle membrane excitability (Stewart et al. 2007). This is caused by an increase in the activity of the Na+/K+-pump through glucose (Green et al. 2007) and possibly insulin and catecholamines (Clausen 2003). While we measured only small differences in [A]v and none in [NA]v, we observed large differences in [Insulin]v and [glu]cap in our study.
An activation of the Na+/K+ pump reduces the intracellular Na+ content and should, consequently, result in a marked release of sodium from intracellular stores under CHO substitution. This effect can occur in resting and exercising muscles and other inactive tissues. A stronger activation of the Na+/K+ pump under influence of glucose should also result in a reduced [K+]v in the CHO trial compared with the Plac trial. Such an increase in the extracellular K+ during exercise without CHO has been shown in several other investigations (Sjogaard 1990b; Sjogaard et al. 1985). However, in the present study, there were no differences in [K+]v between the trials. This might be due to the fact that inactive tissues like non-working muscles can take up electrolytes like [K+] during exercise (Lindinger et al. 1990). High K+ values occurring during leg exercise, as in the Plac trial of the present study, might be reduced when measured in the antecubital vein by uptake in inactive muscles (Clausen 2010). However, this might also apply to sodium. After cessation of the exercise in our study, the difference in sodium between the trials remained significant, and [Na+] further decreased in the Plac trial. This indicates that exercise-induced sodium shifts are not the only reason for the present data. An osmotic inactivation of sodium as described by Noakes et al. (2005) might occur even after exercise.
Conclusions
The present study is the first to show an influence of CHO on the sodium balance during HIHVT. [Na] +v and PV are regulated independently of each other during short bouts of high-intensity exercise. The results are in contrast to CET studies even when using the same dose of maltodextrin or the same mean exercise intensity. This might be caused by the larger activity of type 2 muscle fibres during the exercise bouts with subsequently larger [Na+] and [K+] fluxes. Influences of glucose supplementation on the sodium balance can be deduced from the investigations of Karelis et al. (2002) and Stewart et al. (2007), showing that the compound action potential is influenced by glucose administration. Our results were obtained with large amounts of CHO. Future studies should investigate whether these results are reproducible with smaller CHO doses. In the present study, we could not identify the responsible mechanisms for the stabilisation of sodium. To reveal these mechanisms, it is necessary to perform further studies with HIHVT and investigate the electrolyte and fluid shifts between different body compartments.
Abbreviations
- […]cap :
-
Capillary concentration of…
- […]v :
-
Venous concentration of…
- A:
-
Adrenaline
- ANOVA:
-
Analysis of variance
- BIA:
-
Bio impedance analysis
- BV:
-
Blood volume
- CET:
-
Continuous endurance training
- CHO:
-
Carbohydrate
- ECW:
-
Extracellular water
- HIHVT:
-
High-intensity high-volume training
- HIIT:
-
High-intensity interval training
- HIT:
-
High-intensity training
- ICW:
-
Intracellular water
- Kg/bw:
-
Kilogram/body weight
- Min:
-
Minute
- NA:
-
Noradrenaline
- Plac:
-
Placebo = water
- PV:
-
Plasma volume
- TBW:
-
Total body water
- VE:
-
Ventilation
References
Anastasiou CA, Kavouras SA, Arnaoutis G, Gioxari A, Kollia M, Botoula E, Sidossis LS (2009) Sodium replacement and plasma sodium drop during exercise in the heat when fluid intake matches fluid loss. J Athl Train 44:117–123
Baker LB, Lang JA, Kenney WL (2009) Change in body mass accurately and reliably predicts change in body water after endurance exercise. Eur J Appl Physiol 105:959–967
Bangsbo J, Madsen K, Kiens B, Richter EA (1996) Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. J Physiol 495(Pt 2):587–596
Burge CM, Skinner SL (1995) Determination of hemoglobin mass and blood volume with CO: evaluation and application of a method. J ApplPhysiol (1985) 79:623–631
Cairns SP, Lindinger MI (2008) Do multiple ionic interactions contribute to skeletal muscle fatigue? J Physiol 586:4039–4054
Clausen T (2003) Na + -K + pump regulation and skeletal muscle contractility. Physiol Rev 83:1269–1324
Clausen T (2010) Hormonal and pharmacological modification of plasma potassium homeostasis. FundamClin Pharmacol 24:595–605
Clifford PS, Hellsten Y (2004) Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol 97:393–403
Cope FW (1967) NMR evidence for complexing of Na+ in muscle, kidney, and brain, and by actomyosin. The relation of cellular complexing of Na+ to water structure and to transport kinetics. J Gen Physiol 50:1353–1375
Coyle EF, Hamilton MT, Alonso JG, Montain SJ, Ivy JL (1991) Carbohydrate metabolism during intense exercise when hyperglycemic. J Appl Physiol (1985) 70:834–840
Criswell D, Powers S, Lawler J, Tew J, Dodd S, Iryiboz Y, Tulley R, Wheeler K (1991) Influence of a carbohydrate-electrolyte beverage on performance and blood homeostasis during recovery from football. Int J Sport Nutr 1:178–191
Criswell D, Renshler K, Powers SK, Tulley R, Cicale M, Wheeler K (1992) Fluid replacement beverages and maintenance of plasma volume during exercise: role of aldosterone and vasopressin. Eur J Appl Physiol Occup Physiol 65:445–451
Davis JM, Lamb DR, Pate RR, Slentz CA, Burgess WA, Bartoli WP (1988) Carbohydrate-electrolyte drinks: effects on endurance cycling in the heat. Am J Clin Nutr 48:1023–1030
De Bock K, Derave W, Ramaekers M, Richter EA, Hespel P (2007) Fiber type-specific muscle glycogen sparing due to carbohydrate intake before and during exercise. J Appl Physiol (1985) 102:183–188
de Sousa MV, Simoes HG, Oshiiwa M, Rogero MM, Tirapegui J (2007) Effects of acute carbohydrate supplementation during sessions of high-intensity intermittent exercise. Eur J Appl Physiol 99:57–63
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37:247–248
Essen B (1978a) Glycogen depletion of different fibre types in human skeletal muscle during intermittent and continuous exercise. Acta Physiol Scand 103:446–455
Essen B (1978b) Studies on the regulation of metabolism in human skeletal muscle using intermittent exercise as an experimental model. Acta Physiol Scand Suppl 454:1–32
Essen B, Hagenfeldt L, Kaijser L (1977) Utilization of blood-borne and intramuscular substrates during continuous and intermittent exercise in man. J Physiol 265:489–506
Fricke G (1965) Über das Verhalten des Zellfaktors bei körperlicher Arbeit. Cardologia 47:25–44
Fritzsche RG, Switzer TW, Hodgkinson BJ, Lee SH, Martin JC, Coyle EF (2000) Water and carbohydrate ingestion during prolonged exercise increase maximal neuromuscular power. J Appl Physiol 88:730–737
Gisolfi CV, Summers RW, Lambert GP, Xia T (1998) Effect of beverage osmolality on intestinal fluid absorption during exercise. J Appl Physiol 85:1941–1948
Gollnick PD, Karlsson J, Piehl K, Saltin B (1974) Selective glycogen depletion in skeletal muscle fibres of man following sustained contractions. J Physiol 241:59–67
Green HJ, Duhamel TA, Holloway GP, Moule JW, Ouyang J, Ranney D, Tupling AR (2007) Muscle Na + -K + -ATPase response during 16 h of heavy intermittent cycle exercise. Am J Physiol Endocrinol Metab 293:E523–E530
Hellsten Y, Nyberg M, Jensen LG, Mortensen SP (2012) Vasodilator interactions in skeletal muscle blood flow regulation. J Physiol 590:6297–6305
Jeukendrup AE, McLaughlin J (2011) Carbohydrate ingestion during exercise: effects on performance, training adaptations and trainability of the gut. Nestle Nutr Inst Workshop Ser 69:1–12
Karelis AD, Peronnet F, Gardiner PF (2002) Glucose infusion attenuates muscle fatigue in rat plantaris muscle during prolonged indirect stimulation in situ. Exp Physiol 87:585–592
Klitzman B, Duling BR (1979) Microvascular hematocrit and red cell flow in resting and contracting striated muscle. Am J Physiol 237:H481–H490
Koulmann N, Jimenez C, Regal D, Bolliet P, Launay JC, Savourey G, Melin B (2000) Use of bioelectrical impedance analysis to estimate body fluid compartments after acute variations of the body hydration level. Med Sci Sports Exerc 32:857–864
Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gomez JM, Heitmann BL, Kent-Smith L, Melchior JC, Pirlich M, Scharfetter H, Schols AM, Pichard C (2004) Bioelectrical impedance analysis–part I: review of principles and methods. Clin Nutr 23:1226–1243
Lamb DR, Brodowicz GR (1986) Optimal use of fluids of varying formulations to minimise exercise-induced disturbances in homeostasis. Sports Med 3:247–274
Leiper JB, Nicholas CW, Ali A, Williams C, Maughan RJ (2005) The effect of intermittent high-intensity running on gastric emptying of fluids in man. Med Sci Sports Exerc 37:240–247
Lindinger MI, Heigenhauser GJ, McKelvie RS, Jones NL (1990) Role of nonworking muscle on blood metabolites and ions with intense intermittent exercise. Am J Physiol 258:R1486–R1494
Lindinger MI, Heigenhauser GJ, McKelvie RS, Jones NL (1992) Blood ion regulation during repeated maximal exercise and recovery in humans. Am J Physiol 262:R126–R136
Lott ME, Hogeman C, Herr M, Gabbay R, Sinoway LI (2007) Effects of an oral glucose tolerance test on the myogenic response in healthy individuals. Am JPhysiol Heart CircPhysiol 292:H304–H310
Maassen N, Busse MW (1989) The relationship between lactic acid and work load: a measure for endurance capacity or an indicator of carbohydrate deficiency? Eur J Appl Physiol Occup Physiol 58:728–737
Maassen N, Vardaxoglou KFM (2006) The impact of highly intensive interval like training on respiration. European College of Sport Science, 11th Annual Congress, Lausanne
Maassen N, Weis T, Schneider G, Schmidt W (1995) Carbohydrate rich beverages reduce plasma volume decrease during endurance exercise. Dehydration, rehydration and exercise in the heat. An International Conference, Nottingham, England. Abstracts of Poster Presentations, 15
Maassen N, Foerster M, Mairbaurl H (1998) Red blood cells do not contribute to removal of K+ released from exhaustively working forearm muscle. J Appl Physiol (1985) 85:326–332
Maassen M, Kummer O, Chouchakov VMN (2007) Die Wirkung von Kohlenhydratgaben während hochintensiver Intervallarbeit auf den Energiestoffwechsel und den Wasserhaushalt. Kummer O Chouchakov V, Maassen N. 7/8. 2007. Deutsche Zeitschrift für Sportmedizin
Maughan RJ, Meyer NL (2013) Hydration during intense exercise training. Nestle Nutr Inst Workshop Ser 76:25–37
Mayer CU, Treff G, Fenske WK, Blouin K, Steinacker JM, Allolio B (2015) High incidence of hyponatremia in rowers during a four-week training camp. Am J Med 128:1144–1151
Michel CC, Curry FE (1999) Microvascular permeability. Physiol Rev 79:703–761
Murray R, Eddy DE, Murray TW, Seifert JG, Paul GL, Halaby GA (1987) The effect of fluid and carbohydrate feedings during intermittent cycling exercise. Med Sci Sports Exerc 19:597–604
Murray R, Seifert JG, Eddy DE, Paul GL, Halaby GA (1989) Carbohydrate feeding and exercise: effect of beverage carbohydrate content. Eur J Appl Physiol Occup Physiol 59:152–158
Murray B, Stofan J, Eichner E (2003) Hyponatremia in athletes. Sports Sci Exch Gatorate Sports Sci Ins 16:88
Nassis GP, Williams C, Chisnall P (1998) Effect of a carbohydrate-electrolyte drink on endurance capacity during prolonged intermittent high intensity running. Br J Sports Med 32:248–252
Nicholas CW, Tsintzas K, Boobis L, Williams C (1999) Carbohydrate-electrolyte ingestion during intermittent high-intensity running. Med Sci Sports Exerc 31:1280–1286
Nielsen B, Sjogaard G, Bonde-Petersen F (1984) Cardiovascular, hormonal and body fluid changes during prolonged exercise. Eur J Appl Physiol OccupPhysiol 53:63–70
Nielsen HB, Febbraio MA, Ott P, Krustrup P, Secher NH (2007) Hepatic lactate uptake versus leg lactate output during exercise in humans. J Appl Physiol (1985) 103:1227–1233
Noakes TD, Sharwood K, Speedy D, Hew T, Reid S, Dugas J, Almond C, Wharam P, Weschler L (2005) Three independent biological mechanisms cause exercise-associated hyponatremia: evidence from 2,135 weighed competitive athletic performances. Proc Natl Acad Sci USA 102:18550–18555
O’Brien C, Young AJ, Sawka MN (2002) Bioelectrical impedance to estimate changes in hydration status. Int J Sports Med 23:361–366
Petersen HA, Fueger PT, Bracy DP, Wasserman DH, Halseth AE (2003) Fiber type-specific determinants of Vmax for insulin-stimulated muscle glucose uptake in vivo. Am J Physiol Endocrinol Metab 284:E541–E548
Poole DC (2014) Crosstalk opposing view: de novo capillary recruitment in healthy muscle is not necessary to explain physiological outcomes. J Physiol 592:5133–5135
Rippe B (2008) Free water transport, small pore transport and the osmotic pressure gradient three-pore model of peritoneal transport. Nephrol Dial Transplant 23:2147–2153
Rose AJ, Richter EA (2005) Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology (Bethesda) 20:260–270
Ruff RL (1996) Sodium channel slow inactivation and the distribution of sodium channels on skeletal muscle fibres enable the performance properties of different skeletal muscle fibre types. Acta Physiol Scand 156:159–168
Sanders B, Noakes TD, Dennis SC (2001) Sodium replacement and fluid shifts during prolonged exercise in humans. Eur J Appl Physiol 84:419–425
Schmidt W, Prommer N (2005) The optimised CO-rebreathing method: a new tool to determine total haemoglobin mass routinely. Eur Appl Physiol 95:486–495
Schmidt W, Maassen N, Trost F, Boning D (1988) Training induced effects on blood volume, erythrocyte turnover and haemoglobin oxygen binding properties. Eur J Appl Physiol Occup Physiol 57:490–498
Shi X, Gisolfi CV (1998) Fluid and carbohydrate replacement during intermittent exercise. Sports Med 25:157–172
Shi X, Passe DH (2010) Water and solute absorption from carbohydrate-electrolyte solutions in the human proximal small intestine: a review and statistical analysis. Int J Sport Nutr Exerc Metab 20:427–442
Shi X, Summers RW, Schedl HP, Flanagan SW, Chang R, Gisolfi CV (1995) Effects of carbohydrate type and concentration and solution osmolality on water absorption. Med Sci Sports Exerc 27:1607–1615
Shushakov V, Stubbe C, Peuckert A, Endeward V, Maassen N (2007) The relationships between plasma potassium, muscle excitability and fatigue during voluntary exercise in humans. ExpPhysiol 92:705–715
Sjogaard G (1990a) Exercise-induced muscle fatigue: the significance of potassium. Acta Physiol ScandSuppl 593:1–63
Sjogaard G (1990b) Exercise-induced muscle fatigue: the significance of potassium. Acta Physiol Scand Suppl 593:1–63
Sjogaard G, Saltin B (1982) Extra- and intracellular water spaces in muscles of man at rest and with dynamic exercise. Am J Physiol 243:R271–R280
Sjogaard G, Adams RP, Saltin B (1985) Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol 248:R190–R196
Stewart RD, Duhamel TA, Foley KP, Ouyang J, Smith IC, Green HJ (2007) Protection of muscle membrane excitability during prolonged cycle exercise with glucose supplementation. J Appl Physiol 103:331–339
Thomsen JK, Fogh-Andersen N, Bulow K, Devantier A (1991) Blood and plasma volumes determined by carbon monoxide gas, 99mTc-labelled erythrocytes, 125I-albumin and the T 1824 technique. Scand J Clin Lab Invest 51:185–190
Titze J (2014) Sodium balance is not just a renal affair. Curr Opin Nephrol Hypertens 23:101–105
Titze J (2015) A different view on sodium balance. Curr Opin Nephrol Hypertens 24:14–20
Tsintzas K, Simpson EJ, Seevaratnam N, Jones S (2003) Effect of exercise mode on blood glucose disposal during physiological hyperinsulinaemia in humans. Eur J Appl Physiol 89:217–220
Van Beaumont W, Greenleaf JE, Juhos L (1972) Disproportional changes in hematocrit, plasma volume, and proteins during exercise and bed rest. J Appl Physiol 33:55–61
Vrijens DM, Rehrer NJ (1999) Sodium-free fluid ingestion decreases plasma sodium during exercise in the heat. J Appl Physiol (1985) 86:1847–1851
Acknowledgments
We thank Hannelore Konrad for her invaluable technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report that there were no conflicts of interest during this study.
Additional information
Communicated by Michael Lindinger.
Rights and permissions
About this article
Cite this article
Schrader, M., Treff, B., Sandholtet, T. et al. Carbohydrate supplementation stabilises plasma sodium during training with high intensity. Eur J Appl Physiol 116, 1841–1853 (2016). https://doi.org/10.1007/s00421-016-3429-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3429-4