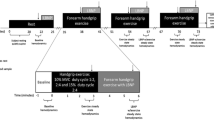
Abstract
Purpose
The effect of acute activation of the sympathetic nervous system on the dynamic response of muscle hyperaemia during exercise at different intensities is not clear.
Methods
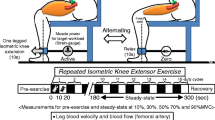
To explore this, six men performed 16, 5-min bouts of intermittent calf contractions at two intensities (25 and 50 % MVC) and two levels of sympathetic activation (CPT cold pressor test, CON control). Mean arterial pressure (MAP) and leg vascular conductance (LVC leg blood flow/MAP) were measured during rest and contractions (3 s intervals), and dynamic response characteristics of LVC were estimated using curve-fitting and empirical modeling.
Results
MAP was ~20 % greater (P ≤ 0.05) during CPT than CON before and during initial contractions at both intensities. At 25 % MVC, CPT reduced the exercise-induced change in LVC (0.109 vs 0.125 ml 100 ml−1 min−1 mmHg−1; P < 0.05), an effect attributed to the reduction in the amplitude of the fast growth phase (0.091 vs 0.128 1 ml 100 ml−1 min−1 mmHg−1; P < 0.05). At 50 % MVC, CPT also blunted the fast growth phase (0.147 vs 0.189 ml 100 ml−1 min−1 mmHg−1; P < 0.05), but the total change in LVC during exercise was unaffected because of a significant reduction in the amplitude of the rapid decay phase and tendency (P = 0.1) for a lower amplitude of the slow decay phase.
Conclusion
Increased sympathetic constraint of vasodilation persists during initial contractions but is overcome at the high intensity by a mechanism apparently related to hyperaemic decay.






Similar content being viewed by others
Abbreviations
- A n :
-
Amplitude of phase n
- CON:
-
Control
- CON25:
-
Control condition at 25 % MVC
- CON50:
-
Control condition at 50 % MVC
- CPT:
-
Cold pressor test
- CPT25:
-
Cold pressor test at 25 % MVC
- CPT50:
-
Cold pressor test at 50 % MVC
- EMG:
-
Electromyogram
- F n :
-
Conditional argument for phase n
- GM:
-
Gastrocnemius lateralis
- LBF:
-
Leg blood flow
- LVC:
-
Leg vascular conductance
- MAP:
-
Mean arterial pressure
- MVC:
-
Maximum voluntary contraction
- T:
-
Temperature
- TA:
-
Tibialis anterior
- TDn :
-
Time delay of phase n
- τn :
-
Time constant of phase n
References
Andersen P, Adams RP, Sjogaard G, Thorboe A, Saltin B (1985) Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol 59:1647–1653
Boulton D, Taylor CE, Macefield VG, Green S (2014) Effect of contraction intensity on sympathetic nerve activity to active human skeletal muscle. Front Physiol 5:1–9
Buckwalter JB, Clifford PS (1999) alpha-adrenergic vasoconstriction in active skeletal muscles during dynamic exercise. Am J Physiol (Heart Circ Physiol) 277:H33–H39
Buckwalter JB, Clifford PS (2001) The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exerc Sports Sci Rev 29:159–163
Buckwalter JB, Naik JS, Valic Z, Clifford PS (2001) Exercise attenuates alpha-adrenergic-receptor responsiveness in skeletal musle vasculature. J Appl Physiol 90:172–178
Dinenno FA, Joyner MJ (2003) Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol 553(1):281–292
Dinenno FA, Joyner MJ (2004) Combined NO and PG inhibition augments alpha-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol (Heart Circ Physiol) 287:H2576–H2584
Dinenno FA, Masuki S, Joyner MJ (2005) Impaired modulation of sympathetic alpha-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol 567:311–321
Donald DE, Rowlands DJ, Ferguson DA (1970) Similarity of blood flow in the normal and the sympathectomised dog hind limb during graded exercise. Circ Res 25:185–199
Donnelly J, Green S (2013) Effect of hypoxia on the dynamic response characteristics of hyperaemia in the contracting human calf muscle. Exp Physiol 98(1):81–93
Egana M, Green S (2005) Effect of body tilt on calf muscle performance and blood flow in humans. J Appl Physiol 98:2249–2258
Fadel PJ, Farias M III, Gallagher KM, Wang Z, Thomas GD (2012) Oxidative stress and enhanced sympathetic vasoconstriction in contracting muscles of nitrate-tolerant rats and humans. J Physiol 590(2):395–407
Fitzpatrick R, Burke D, Gandevia SC (1996) Loop gain of reflexes controlling human standing measured with the use of postural and vestibular disturbances. J Neurophysiol 76:3994–4008
Green S, Thorp R, Reeder E, Donnelly J, Fordy G (2011) Venous occlusion plethysmography versus Doppler ultrasound in the assessment of leg blood flow during calf exercise. Eur J Appl Physiol 111:1889–1900
Hamann JJ, Buckwalter JB, Valic Z, Clifford PS (2002) Sympathetic constraint of muscle blood flow at the onset of dynamic exercise. J Appl Physiol 92:2452–2456
Ives SJ, Andtbacka RHI, Noyes RD, Morgan RG, Gifford JR, Park S-Y, Symons JD, Richardson RS (2013) alpha1-adrenergic responsiveness in human skeletal muscle feed arteries: the impact of reducing extracellular pH. Exp Physiol 98(1):256–267
Jendzjowsky NG, DeLorey DS (2013) Short-term exercise training enhances functional sympatholysis through a nitric oxide-dependent mechanism. J Physiol 591(6):1535–1549
Joyner MJ, Lennon RL, Wedel DJ, Rose SH, Shepherd JT (1990) Blood flow to contracting human muscles: influence of increased sympathetic activity. J Appl Physiol 68:1453–1457
Kagaya A, Saito M, Ogita F, Shinohara M (1994) Exhausting handgrip exercise reduces the blood flow in the active calf muscle exercising at low intensity. Eur J Appl Physiol 68:252–257
Keller DM, Ogoh S, Greene S, Olivencia-Yurvati A, Raven PB (2004) Inhibition of KATP channel activity augments baroreflex-mediated vasoconstriction in exercising human skeletal muscle. J Physiol 561:273–282
Kiely C, O’Connor E, O’Shea D, Green S, Egana M (2014) Haemodynamic responses during graded and constant-load plantar flexion exercise in middle-aged men and women with type 2 diabetes. J Appl Physiol 117:755–764
Kregel KC, Seals DR, Callister R (1992) Sympathetic nervous system activity during skin cooling in humans: relationship to stimulus intensity and pain sensation. J Physiol 454:359–371
MacAnaney O, Reilly H, O’Shea D, Egana M, Green S (2011) Effect of type 2 diabetes on the dynamic response characteristics of leg vascular conductance during exercise. Diab Vasc Dis Res 8:12–21
Masuki S, Nose H (2003) Arterial baroreflex control of muscle blood flow at the onset of voluntary locomotion in mice. J Physiol 553(1):191–201
Mortensen SP, Morkeberg J, Thaning P, Hellsten Y, Saltin B (2012a) Two weeks of muscle immobilization impairs functional sympatholysis but increases exercise hyperemia and the vasodilatory responsiveness to infused ATP. Am J Physiol (Heart Circ Physiol) 302:H2074–H2082
Mortensen SP, Nyberg M, Winding K, Saltin B (2012b) Lifelong physical activity preserves functional sympatholysis and purinergic signalling in the ageing human leg. J Physiol 590(23):6227–6236
Peterson DF, Armstrong RB, Laughlin MH (1988) Sympathetic neural influences on muscle blood flow in rats during submaximal exercise. J Appl Physiol 65:434–440
Reeder E, Green S (2012) Dynamic response characteristics of muscle hyperaemia: effect of exercise intensity and relation to electromyographic activity. Eur J Appl Physiol 112:3997–4013
Remensnyder JP, Mitchell JH, Sarnoff SJ (1962) Functional sympatholysis during muscular activity. observations on influence of carotid sinus on oxygen uptake. Circ Res 11:370–380
Saitou K, Masuda T, Michikami D, Kojima R, Okada M (2000) Innervation zones of the upper and lower limb muscles estimated by using multichannel surface EMG. J Human Ergol 29:35–52
Saltin B (2007) Exercise hyperaemia: magnitude and aspects on regulation in humans. J Physiol 583(3):819–823
Saunders NR, Pyke KE, Tschakovsky ME (2005) Dynamic response characteristics of local muscle blood flow regulatory mechanisms in human forearm exercise. J Appl Physiol 98:1286–1296
Savard G, Strange S, Kiens B, Richter EA, Christensen NJ, Saltin B (1987) Noradrenaline spillover during exercise in active versus resting skeletal muscle in man. Acta Physiol Scand 131:507–515
Sprangers RLH, Wesseling KH, Imholz ALT, Imholz BPM, Wieling W (1991) Initial blood pressure fall on stand up and exercise explained by changes in total peripheral resistance. J Appl Physiol 70:523–530
Thaning P, Bune LT, Zaar M, Saltin B, Rosenmeier JB (2011) Functional sympatholysis during exercise in patients with type 2 diabetes with intact response to acetylcholine. Diab Care 34:1186–1191
Thomas GD, Segal SS (2004) Neural control of blood flow during exercise. J Appl Physiol 97:731–738
Thomas GD, Hansen J, Victor RG (1994) Inhibition of alpha2-adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hindlimb muscle. Am J Physiol (Heart Circ Physiol) 266:H920–H929
Tschakovsky ME, Hughson RL (1999) Ischemic muscle chemoreflex response elevates blood flow in nonischemic exercising human forearm muscle. Am J Physiol (Heart Circ Physiol) 277:H635–H642
Tschakovsky ME, Hughson RL (2003) Rapid blunting of sympathetic vasoconstriction in the human forearm at the onset of exercise. J Appl Physiol 94:1785–1792
Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ (2002) Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol 541:623–635
Vongpatanasin W, Wang Z, Arbique D, Arbique G, Adams-Huet B, Mitchell JH, Victor RG, Thomas GD (2011) Functional sympatholysis is impaired in hypertensive humans. J Physiol 589(5):1209–1220
Watanabe H, Watanabe K, Wadazumi T, Yoneyama F (2007) Effect of exercise intensity on mild rhythmic-handgrip-exercise-induced functional sympatholysis. J Physiol Anthropol 26:593–597
Wieling W, Harms MPM, ten Harkel ADJ, Van Lieshout JJ, Sprangers RLH (1996) Circulatory response evoked by a 3 s bout of dynamic leg exercise in humans. J Physiol 494(2):601–611
Wilkins BW, Schrage WG, Liu Z, Hancock KC, Joyner MJ (2006) Systemic hypoxia and vasoconstrictor responsiveness in exercising human muscle. J Appl Physiol 101:1343–1350
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Keith Phillip George.
Rights and permissions
About this article
Cite this article
Green, S., Cameron, E. Interactive effect of acute sympathetic activation and exercise intensity on the dynamic response characteristics of vascular conductance in the human calf muscle. Eur J Appl Physiol 115, 879–890 (2015). https://doi.org/10.1007/s00421-014-3069-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-014-3069-5