Abstract
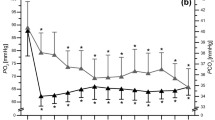
This investigation explored the influence of supplemental oxygen administered during the recovery periods of an interval-based running session on the post-exercise markers of reactive oxygen species (ROS) and inflammation. Ten well-trained male endurance athletes completed two sessions of 10 × 3 min running intervals at 85 % of the maximal oxygen consumption velocity (vVO2peak) on a motorised treadmill. A 90-s recovery period was given between each interval, during which time the participants were administered either a hyperoxic (HYP) (Fraction of Inspired Oxygen (FIO2) 99.5 %) or normoxic (NORM) (FIO2 21 %) gas, in a randomized, single-blind fashion. Pulse oximetry (SpO2), heart rate (HR), blood lactate (BLa), perceived exertion (RPE), and perceived recovery (TQRper) were recorded during each trial. Venous blood samples were taken pre-exercise, post-exercise and 1 h post-exercise to measure Interleukin-6 (IL-6) and Isoprostanes (F2-IsoP). The SpO2 was significantly lower than baseline following all interval repetitions in both experimental trials (p < 0.05). The SpO2 recovery time was significantly quicker in the HYP when compared to the NORM (p < 0.05), with a trend for improved perceptual recovery. The IL-6 and F2-IsoP were significantly elevated immediately post-exercise, but had significantly decreased by 1 h post-exercise in both trials (p < 0.05). There were no differences in IL-6 or F2-IsoP levels between trials. Supplemental oxygen provided during the recovery periods of interval based exercise improves the recovery time of SPO2 but has no effect on post-exercise ROS or inflammatory responses.


Similar content being viewed by others
References
Alessio HM (1993) Exercise-induced oxidative stress. Med Sci Sports Exerc 25:218–224
Ali M, Schlidt S, Chandel N, Hynes K, Schumacker P, Gewertz B (1999) Endothelial permiability and IL-6 production during hypoxia: role of ROS in signal transduction. Am J Physiol 5:1057–1065
Allen PD, Pandolf KB (1977) Perceived exertion associated with breathing hyperoxic mixtures during supramaximal work. Med Sci Sports Exerc 9:122–127
Borg G (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A (2006) Biomarkers of oxidative damage in human disease. Clin Chem 52:601–623
Dempsey JA, Wagner PD (1999) Exercise-induced arterial hypoxemia. J Appl Physiol 87:1997–2006
Fallon K (2001) The acute phase response and exercise: the ultra-marathon as prototype exercise. Clin J Sport Med 11:38–43
Jones AM, Doust JH (1996) A 1 % treadmill grade most accurately reflects the energetic cost of outdoor running. J Sports Sci 14:321–327
Kay B, Stannard S, Morton R, North N (2008) Hyperoxia during recovery improves peak power during repeated wingate cycle performance. Braz J Biomotricity 2:92–100
Kentta G, Hassmen P (1998) Overtraining and recovery—A conceptual model. Sports Med 26:1–16
Levine RL, Stadtman ER (2001) Oxidative modification of proteins during aging. Exp Gerontol 36:1495–1502
Maeda T, Yasukouchi A (1998) Blood lactate disappearance during breathing hyperoxic gas after exercise in two different physical fitness groups on the workload fixed at 130 % AT. Appl Human Sci J Physiol Anthrop 17:33–40
Mastaloudis A, Morrow JD, Hopkins DW, Devaraj MG (2004) Antioxidant supplementation prevents exercise-induced lipid peroxidation, but not inflammation, in ultramarathon runners. Free Radic Biol Med 2004:1329–1341
Mori TA, Croft KD, Puddey IB, Beilin LJ (1999) An improved method for the measurement of urinary and plasma F2-Isoprostanes using gas chromatography–mass spectrometry. Anal Biochem 268:117–125
Nieman DC, Henson DA, McAnulty SR, McAnulty LS, Morrow JD, Ahmed A, Heward CB (2004) Vitamin E and immunity after the Kona Triathlon World Championship. Med Sci Sports Exerc 36:1328–1335
Niess AM, Hartmann A, Grunert-Fuchs M, Poch B, Speit G (1996) DNA damage after exhaustive treadmill running in trained and untrained men. Int J Sports Med 17:397–403
Nummela A, Hamalainen I, Rusko H (2002) Effect of hyperoxia on metabolic responses and recovery in intermittent exercise. Scand J Med Sci Sports 12:309–315
Ostrowski K, Hermann C, Bangash A, Schjerling P, Nielsen JN, Pedersen BK (1998) A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J Physiol 513:889–894
Peeling P, Andersson R (2011) Effect of hyperoxia during the rest periods of interval training on perceptual recovery and oxygen re-saturation time. J Sports Sci 29:147–150
Perry CG, Talanian JL, Heigenhauser GJ, Spriet LL (2007) The effects of training in hyperoxia vs. normoxia on skeletal muscle enzyme activities and exercise performance. J Appl Physiol 102:1022–1027
Pyne DB (1994) Exercise-induced muscle damage and inflammation: a review. Aust J Sci Med Sport 26:49–58
Romer LM, Dempsey JA (2006) Effects of exercise-induced arterial hypoxemia on limb muscle fatigue and performance. Clin Exp Pharmacol Physiol 33:391–394
Sim M, Dawson B, Landers G, Wiegerinck E, Swinkels D, Townsend M, Trinder D, Peeling P (2012) The effects of carbohydrate ingestion during endurance running on post-exercise inflammation and hepcidin levels. Eur J Appl Physiol 112:1889–1898
van Helvoort HAC, Heijdar YF, Heunks LMA, Meijer PLM, Ruiten W, Thijs HMH, Dekhuijzen PNR (2006) Supplemental oxygen prevents exercise enduced oxidative stress in muscle-wasted paitents with chronic obstructive pulmonary disease. Am J Resp Crit Care Med 173:1122–1129
Wilber RL, Holm PL, Morris DM, Dallam GM, Subudhi AW, Murray DM, Callam SD (2004) Effect of FIO2 on oxidative stress during interval training at moderate altitude. Med Sci Sports Exerc 36:1888–1894
Acknowledgments
The authors wish to acknowledge the grant funding received from the University of Western Australia’s Research Grant Scheme.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Fabio Fischetti.
Rights and permissions
About this article
Cite this article
White, J., Dawson, B., Landers, G. et al. Effect of supplemental oxygen on post-exercise inflammatory response and oxidative stress. Eur J Appl Physiol 113, 1059–1067 (2013). https://doi.org/10.1007/s00421-012-2521-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-012-2521-7