Abstract
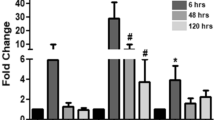
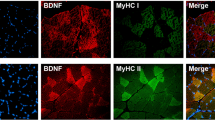
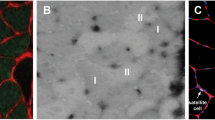
The contribution of neuronal nitric oxide synthase (nNOS) to angiogenesis in human skeletal muscle after endurance exercise is controversially discussed. We therefore ascertained whether the expression of nNOS is associated with the capillary density in biopsies of the vastus lateralis (VL) muscle that had been derived from 10 sedentary male subjects before and after moderate training (four 30-min weekly jogging sessions for 6 months, with a heart-rate corresponding to 75% VO2max). In these biopsies, nNOS was predominantly expressed as alpha-isoform with exon-mu and to a lesser extent without exon-mu, as determined by RT-PCR. The mRNA levels of nNOS were quantified by real-time PCR and related to the capillary-to-fibre ratio and the numerical density of capillaries specified by light microscopy. If the VL biopsies of all subjects were co-analysed, mRNA levels of nNOS were non-significantly elevated after training (+34%; P > 0.05). However, only five of the ten subjects exhibited significant (P ≤ 0.05) elevations in the capillary-to-fibre ratio (+25%) and the numerical density of capillaries (+21%) and were thus undergoing angiogenesis. If the VL biopsies of these five subjects alone were evaluated, the mRNA levels of nNOS were significantly up-regulated (+128%; P ≤ 0.05) and correlated positively (r = 0.8; P ≤ 0.01) to angiogenesis. Accordingly, nNOS protein expression in VL biopsies quantified by immunoblotting was significantly increased (+82%; P ≤ 0.05) only in those subjects that underwent angiogenesis. In conclusion, the expression of nNOS at mRNA and protein levels was statistically linked to capillarity after exercise suggesting that nNOS is involved in the angiogenic response to training in human skeletal muscle.




Similar content being viewed by others
References
Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM (2008) HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451:1008–1012
Baum O, Da Silva-Azevedo L, Willerding G, Wöckel A, Planitzer G, Gossrau R, Pries AR, Zakrzewicz A (2004) Endothelial NOS is main mediator for shear stress-dependent angiogenesis in skeletal muscle after prazosin administration. Am J Physiol Heart Circ Physiol 287:H2300–H2308
Bouchard C, Rankinen T (2001) Individual differences in response to regular physical activity. Med Sci Sports Exerc 33:446–451
Bradley SJ, Kingwell BA, Canny BJ, McConell GK (2007) Skeletal muscle neuronal nitric oxide synthase micro protein is reduced in people with impaired glucose homeostasis and is not normalized by exercise training. Metabolism 56:1405–1411
Bray MS, Hagberg JM, Perusse L, Rankinen T, Roth SM, Wolfarth B, Bouchard C (2009) The human gene map for performance and health-related fitness phenotypes: the 2006–2007 update. Med Sci Sports Exerc 41:35–73
Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z (2009) The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci U S A 106:21401–21406
Coffey VG, Hawley JA (2007) The molecular bases of training adaptation. Sports Med 37:737–763
Copp SW, Hirai DM, Schwagerl PJ, Musch TI, Poole DC (2010) Effects of neuronal nitric oxide synthase inhibition on resting and exercising hindlimb muscle blood flow in the rat. J Physiol 588:1321–1331
Da Silva-Azevedo L, Baum O, Zakrzewicz A, Pries AR (2002) Vascular endothelial growth factor is expressed in endothelial cells isolated from skeletal muscles of nitric oxide synthase knockout mice during prazosin-induced angiogenesis. Biochem Biophys Res Commun 297:1270–1276
Da Silva-Azevedo L, Jahne S, Hoffmann C, Stalder D, Heller M, Pries AR, Zakrzewicz A, Baum O (2009) Up-regulation of the peroxiredoxin-6 related metabolism of reactive oxygen species in skeletal muscle of mice lacking neuronal nitric oxide synthase. J Physiol 587:655–668
Egginton S (2009) Invited review: activity-induced angiogenesis. Pflugers Arch 457:963–977
Frandsen U, Hoffner L, Betak A, Saltin B, Bangsbo J, Hellsten Y (2000) Endurance training does not alter the level of neuronal nitric oxide synthase in human skeletal muscle. J Appl Physiol 89:1033–1038
Gavin TP (2009) Basal and exercise-induced regulation of skeletal muscle capillarization. Exerc Sport Sci Rev 37:86–92
Gustafsson T, Rundqvist H, Norrbom J, Rullman E, Jansson E, Sundberg CJ (2007) The influence of physical training on the angiopoietin and VEGF-A systems in human skeletal muscle. J Appl Physiol 103:1012–1020
Hoppeler H, Howald H, Conley K, Lindstedt SL, Claassen H, Vock P, Weibel ER (1985) Endurance training in humans: aerobic capacity and structure of skeletal muscle. J Appl Physiol 59:320–327
Hoppeler H, Klossner S, Fluck M (2007) Gene expression in working skeletal muscle. Adv Exp Med Biol 618:245–254
Hudlicka O (1998) Is physiological angiogenesis in skeletal muscle regulated by changes in microcirculation? Microcirculation 5:7–23
Hudlicka O, Brown MD (2009) Adaptation of skeletal muscle microvasculature to increased or decreased blood flow: role of shear stress, nitric oxide and vascular endothelial growth factor. J Vasc Res 46:504–512
Hudlicka O, Brown MD, Silgram H (2000) Inhibition of capillary growth in chronically stimulated rat muscles by N(G)-nitro-l-arginine, nitric oxide synthase inhibitor. Microvasc Res 59:45–51
Jackson MJ, Pye D, Palomero J (2007) The production of reactive oxygen and nitrogen species by skeletal muscle. J Appl Physiol 102:1664–1670
Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, Parikh SV, Weiss RM, Chamberlain JS, Moore SA, Campbell KP (2008) Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature 456:511–515
Kobzik L, Reid MB, Bredt DS, Stamler JS (1994) Nitric oxide in skeletal muscle. Nature 372:546–548
Laine R, de Montellano PR (1998) Neuronal nitric oxide synthase isoforms alpha and mu are closely related calpain-sensitive proteins. Mol Pharmacol 54:305–312
Lau KS, Grange RW, Isotani E, Sarelius IH, Kamm KE, Huang PL, Stull JT (2000) nNOS and eNOS modulate cGMP formation and vascular response in contracting fast-twitch skeletal muscle. Physiol Genomics 2:21–27
Leick L, Hellsten Y, Fentz J, Lyngby SS, Wojtaszewski JF, Hidalgo J, Pilegaard H (2009) PGC-1alpha mediates exercise-induced skeletal muscle VEGF expression in mice. Am J Physiol Endocrinol Metab 297:E92–E103
McConell GK, Bradley SJ, Stephens TJ, Canny BJ, Kingwell BA, Lee-Young RS (2007) Skeletal muscle nNOS mu protein content is increased by exercise training in humans. Am J Physiol Regul Integr Comp Physiol 293:R821–R828
Melikian N, Seddon MD, Casadei B, Chowienczyk PJ, Shah AM (2009) Neuronal nitric oxide synthase and human vascular regulation. Trends Cardiovasc Med 19:256–262
Percival JM, Anderson KN, Gregorevic P, Chamberlain JS, Froehner SC (2008) Functional deficits in nNOSmu-deficient skeletal muscle: myopathy in nNOS knockout mice. PLoS One 3:e3387
Rudnick J, Puttmann B, Tesch PA, Alkner B, Schoser BG, Salanova M, Kirsch K, Gunga HC, Schiffl G, Luck G, Blottner D (2004) Differential expression of nitric oxide synthases (NOS 1–3) in human skeletal muscle following exercise countermeasure during 12 weeks of bed rest. FASEB J 18:1228–1230
Salanova M, Schiffl G, Puttmann B, Schoser BG, Blottner D (2008) Molecular biomarkers monitoring human skeletal muscle fibres and microvasculature following long-term bed rest with and without countermeasures. J Anat 212:306–318
Sarelius I, Pohl U (2010) Control of muscle blood flow during exercise: local factors and integrative mechanisms. Acta Physiol (Oxf) 199:349–365
Silvagno F, Xia H, Bredt DS (1996) Neuronal nitric-oxide synthase-mu, an alternatively spliced isoform expressed in differentiated skeletal muscle. J Biol Chem 271:11204–11208
Stamler JS, Meissner G (2001) Physiology of nitric oxide in skeletal muscle. Physiol Rev 81:209–237
Suter E, Hoppeler H, Claassen H, Billeter R, Aebi U, Horber F, Jaeger P, Marti B (1995) Ultrastructural modification of human skeletal muscle tissue with 6-month moderate-intensity exercise training. Int J Sports Med 16:160–166
Timmons JA, Sundberg CJ (2006) Oligonucleotide microarray expression profiling: human skeletal muscle phenotype and aerobic exercise training. IUBMB Life 58:15–24
Vassilakopoulos T, Deckman G, Kebbewar M, Rallis G, Harfouche R, Hussain SN (2003) Regulation of nitric oxide production in limb and ventilatory muscles during chronic exercise training. Am J Physiol Lung Cell Mol Physiol 284:L452–L457
Wang Y, Newton DC, Robb GB, Kau CL, Miller TL, Cheung AH, Hall AV, VanDamme S, Wilcox JN, Marsden PA (1999) RNA diversity has profound effects on the translation of neuronal nitric oxide synthase. Proc Natl Acad Sci U S A 96:12150–12155
Wehling-Henricks M, Oltmann M, Rinaldi C, Myung KH, Tidball JG (2009) Loss of positive allosteric interactions between neuronal nitric oxide synthase and phosphofructokinase contributes to defects in glycolysis and increased fatigability in muscular dystrophy. Hum Mol Genet 18:3439–3451
Williams JL, Cartland D, Hussain A, Egginton S (2006) A differential role for nitric oxide in two forms of physiological angiogenesis in mouse. J Physiol 570:445–454
Acknowledgments
This study was funded by grants from the Swiss National Science Foundation (SNF, 320030-120269) and the Swiss Foundation for Research on Muscle Diseases (SSEM). We would like to thank Franziska Graber and Adolfo Adriozola for their skilful technical support, and Matthias Müller and Fabio Breil for their helpful discussions.
Conflict of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Martin Flueck.
Rights and permissions
About this article
Cite this article
Huber-Abel, F.A.M., Gerber, M., Hoppeler, H. et al. Exercise-induced angiogenesis correlates with the up-regulated expression of neuronal nitric oxide synthase (nNOS) in human skeletal muscle. Eur J Appl Physiol 112, 155–162 (2012). https://doi.org/10.1007/s00421-011-1960-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-1960-x