Abstract
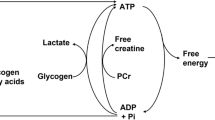
Exercise is the example par excellence of the body functioning as a physiological system. Conventionally we think of the O2 transport process as a major manifestation of that system linking and integrating pulmonary, cardiovascular, hematological and skeletal muscular contributions to the task of getting O2 from the air to the mitochondria, and this process has been well described. However, exercise invokes system responses at levels additional to those of macroscopic O2 transport. One such set of responses appears to center on muscle intracellular PO2, which falls dramatically from rest to exercise. At rest, it approximates 4 kPa, but during heavy endurance exercise it falls to about 0.4–0.5 kPa, an amazingly low value for a tissue absolutely dependent on the continual supply of O2 to meet very high energy demands. One wonders why intracellular PO2 is allowed to fall to such levels. The proposed answer, to be presented in the review, is that a low intramyocyte PO2 is pivotal in: (a) optimizing oxygen’s own physiological transport, and (b) stimulating adaptive gene expression that, after translation, enables greater exercise capacity—all the while maintaining PO2 at levels sufficient to allow oxidative phosphorylation to operate sufficiently fast enough to support intense muscle contraction. Thus, during exercise, reductions of intracellular PO2 to less than 1% of that in the atmosphere enables an integrated response that fundamentally and simultaneously optimizes physiological, biochemical and molecular events that support not only the exercise as it happens but the adaptive changes to increase exercise capacity over the longer term.



Similar content being viewed by others
References
Alders DJC, Groeneveld ABJ, de Kanter FJJ, Van Beek JHGM (2004) Myocardial O2 consumption in porcine left ventricle is heterogeneously distributed in parallel to heterogeneous O2 delivery. Am J Physiol Heart Circ Physiol 287:H1353–H1361
Allen BW, Stamler JS, Piantadosi C (2009) Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation. Trends Mol Med 15:452–460
Berne RM (1963) Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol 204:317–322
Breen EC, Johnson EC, Wagner H, Tseng H-M, Sung LA, Wagner PD (1996) Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol 81:355–361
Cannon WB (1932) Wisdom of the body. Peter Smith, Gloucester, MA
Cardus J, Marrades RM, Roca J, Barberá JA, Diaz O, Masclans JR, Rodriguez-Roisin R, Wagner PD (1998) Effects of FIO2 on leg VO2 during cycle ergometry in sedentary subjects. Med Sci Sports Exerc 30:697–703
Carrier O, Walker JR, Guyton AC (1964) Role of oxygen in autoregulation of blood flow in isolated vessels. Am J Physiol 206:951–954
Clanton TL (2007) Hypoxia-induced reactive oxygen species formation in skeletal muscle. J Appl Physiol 102:2379–2388
Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ (1996) Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem 271:736–741
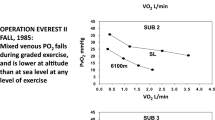
Cymerman A, Reeves JT, Sutton JR, Rock PB, Groves BM, Malconian MK, Young PM, Wagner PD, Houston CS (1989) Operation Everest II: maximal oxygen uptake at extreme altitude. J Appl Physiol 66:2446–2453
Gayeski TEJ, Honig CR (1986) O2 gradients from sarcolemma to cell interior in a red muscle at maximal VO2. Am J Physiol 251:789–799
Gayeski TEJ, Honig CR (1988) Intracellular PO2 in long axis of individual fibers in working dog gracilis muscle. Am J Physiol 254:H1179–H1186
Gonzalez NC, Wood JG (2010) Alveolar hypoxia-induced systemic inflammation: what low PO2 does and does not do. Adv Exp Med Biol 662:27–32
Groebe K, Thews G (1988) Theoretical analysis of oxygen supply to contracted skeletal muscle. Adv Exp Med Biol 222:25–35
Jobsis FF (1977) Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198:1264–1267
Jue T, Anderson S (1990) 1H NMR observation of tissue myoglobin: an indicator of cellular oxygenation in vivo. Magn Reson Med 13:524–528
Kessler M, Lubbers DW (1966) Aufbau und Anwendungs-moglichkeiten verschiedener PO2-electroden. Pflugers Arch Ges Physiol 291:R82
Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD, Breen EC (2009) Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol 587:1755–1767
Olfert IM, Howlett RA, Wagner PD, Breen EC (2010) Myocyte vascular endothelial growth factor is required for exercise-induced skeletal muscle angiogenesis. Am J Physiol Reg Int Comp Physiol 299:R1059–R1067
Pirnay F, Lamy M, Dujardin J, Deroanne R, Petit M (1972) Analysis of femoral venous blood during maximum exercise. J Appl Physiol 33:289–292
Powers SK, Lawler J, Dempsey J, Dodd JA, Landry G (1989) Effects of incomplete pulmonary gas exchange on VO2max. J Appl Physiol 66:2491–2495
Powers SK, Duarte J, Kavazis AN, Talbert EE (2010) Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol 95:1–9
Pronk M, Tiemessen I, Hupperets MDW, Kennedy B, Powell FL, Hopkins SR, Wagner PD (2003) Persistence of the lactate paradox over 8 weeks at 3800 m. High Alt Med Biol 4:431–443
Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD (1995) Myoglobin O2 desaturation during exercise: evidence of limited O2 transport. J Clin Invest 96:1916–1926
Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J, Wagner PD (1999) Evidence of O2 supply-dependent VO2max in the exercise-trained human quadriceps. J Appl Physiol 86:1048–1053
Richardson RS, Haseler LJ, Nygren AT, Bluml S, Frank LR (2001) Local perfusion and metabolic demand during exercise: a noninvasive MRI method of assessment. J Appl Physiol 91:1845–1853
Richardson RS, Duteil S, Wary C, Wray DW, Hoff J, Carlier PG (2006) Human skeletal muscle intracellular oxygenation: the impact of ambient oxygen availability. J Physiol 571:415–424
Roca J, Hogan MC, Story D, Bebout DE, Haab P, Gonzalez R, Ueno O, Wagner PD (1989) Evidence for tissue diffusion limitation of VO2max in normal humans. J Appl Physiol 67:291–299
Ross JM, Fairchild HM, Weldy J, Guyton AC (1962) Autoregulation of blood flow by oxygen lack. Am J Physiol 202:21–24
Semenza GL (1999) Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol 15:551–578
Semenza GL (2010) Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med 2:336–361
Shephard RJ (1969) A non-linear solution of the oxygen conductance equation. Applications to performance at sea-level, at an altitude of 7350 ft. Int Z Angew Physiol 27:212–225
Subczynski WK, Lukiewicz S, Hyde JS (1986) Murine in vivo L-band ESR spin-label oximetry with a loop-gap. Magn Reson Med 3:747–754
Sullivan SM, Johnson PC (1981) Effect of oxygen on blood flow autoregulation in cat sartorius muscle. Am J Physiol (Heart Circ Physiol) 241:H807–H815
Suzuki K, Nakaji S, Yamada M, Totsuka M, Sato K, Sugawara K (2002) Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol Rev 8:6–48
Tang K, Breen EC, Wagner H, Brutsaert TD, Gassmann M, Wagner PD (2004) HIF and VEGF relationships in response to hypoxia and sciatic nerve stimulation in rat gastrocnemius. Respir Physiol Neurobiol 144:71–80
Ushio-Fukai M, Alexander RW (2004) Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem 264:85–97
Vanderkooi JM, Wilson DF (1986) A new method for measuring oxygen in biological systems. Plenum Press, New York, pp 189–193
Vanderkooi JM, Maniara G, Green TJ, Wilson DF (1987) An optical method for measuring of dioxygen concentration based on quenching of phosphorescence. J Biol Chem 262:5476–5482
Wagner PD (1996) Determinants of maximal oxygen transport and utilization. Annu Rev Physiol 58:21–50
Wagner PD, Araoz M, Boushel R, Calbet JAL, Jessen B, Radegran G, Spielvogel H, Sondergaard H, Wagner H, Saltin B (2002) Pulmonary gas exchange and acid-base state at 5,260 m in high-altitude Bolivians and acclimatized lowlanders. J Appl Physiol 92:1393–1400
Wang Z, Noyszewski EA, Leigh JS (1990) In vivo MRS measurement of deoxymyoglobin in human forearms. Magn Reson Med 14:562–567
Weibel ER (1984) The pathway for oxygen structure and function in the mammalian respiratory system. Harvard University Press, Cambridge, MA
Wilson DF, Erecinska M, Drown C, Silver IA (1977) Effect of oxygen tension on cellular energetics. Am J Physiol 233:C135–C140
Zuo L, Clanton TL (2005) Reactive oxygen species formation in the transition to hypoxia in skeletal muscle. Am J Physiol Cell Physiol 289:C207–C216
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Nigel A.S. Taylor.
Rights and permissions
About this article
Cite this article
Wagner, P.D. Muscle intracellular oxygenation during exercise: optimization for oxygen transport, metabolism, and adaptive change. Eur J Appl Physiol 112, 1–8 (2012). https://doi.org/10.1007/s00421-011-1955-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-1955-7