Abstract
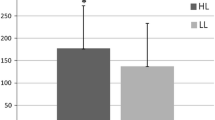
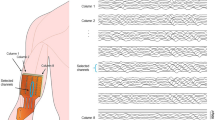
We compared the cardiorespiratory response and muscle recruitment [as determined by electromyography (EMG)] of 37 McArdle patients [19 males, 37.4 ± 2.8 years, body mass index (BMI): 25.1 ± 4.7 kg m−2] and 33 healthy controls (18 males, 36.4 ± 10.0 years, BMI: 25.7 ± 3.8 kg m−2) during cycle-ergometer exercise (an incremental test to exhaustion and a 12-min submaximal constant workload test). We obtained cardiorespiratory [oxygen uptake and heart rate (HR)] and EMG data (rectus femoris and vastus lateralis muscles). During the incremental test, the patients exhibited the expected hyperkinetic cardiovascular response shown by a marked increase in the slope of the HR:Power relationship (p < 0.001). Throughout the incremental test and at the point of fatigue, the patients produced significantly less power than the controls (peak power output: 67 ± 21 vs. 214 ± 56 watts respectively, p < 0.001), yet they demonstrated significantly higher levels of muscle activity for a given absolute power. During the constant workload test, patients displayed higher levels of EMG activity than the controls during the second half of the test, despite a lower power production (34 ± 13 vs. 94 ± 29 watts respectively, p < 0.001). In conclusion, since the McArdle patients required more motor unit recruitment for a given power output, our data suggest that the state of contractility of their muscles is reduced compared with healthy people. Excessive muscle recruitment for a given load could be one of the mechanisms explaining the exercise intolerance of these patients.





Similar content being viewed by others
References
Allen DG, Lamb GD, Westerblad H (2008) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88:287–332
Borg GA (1982) Psychophysical bases of physical exertion. Med Sci Sports Exerc 14:377–381
Brooks GA, Butterfield GE, Wolfe RR, Groves BM, Mazzeo RS, Sutton JR, Wolfel EE, Reeves JT (1991) Increased dependence on blood glucose after acclimatization to 4, 300 m. J Appl Physiol 70:919–927
Chavarren J, Calbet JA (1999) Cycling efficiency and pedalling frequency in road cyclists. Eur J Appl Physiol Occup Physiol 80:555–563
Di Mauro S (2007) Muscle glycogenoses: an overview. Acta Myol 26:35–41
Dirksen RT (2009) Sarcoplasmic reticulum-mitochondrial through-space coupling in skeletal muscle. Appl Physiol Nutr Metab 34:389–395
Farina D, Merletti R, Enoka RM (2004) The extraction of neural strategies from the surface EMG. J Appl Physiol 96:1486–1495
Gonzalez-Freire M, Santiago C, Gomez-Gallego F, Perez M, Foster C, Arenas J, Lucia A (2009) Does the K153R variant of the myostatin gene influence the clinical presentation of women with McArdle disease? Neuromuscul Disord 19:220–222
Haller RG, Lewis SF, Cook JD, Blomqvist CG (1983) Hyperkinetic circulation during exercise in neuromuscular disease. Neurology 33:1283–1287
Haller RG, Clausen T, Vissing J (1998) Reduced levels of skeletal muscle Na + K + -ATPase in McArdle disease. Neurology 50:37–40
Haller RG, Wyrick P, Taivassalo T, Vissing J (2006) Aerobic conditioning: an effective therapy in McArdle’s disease. Ann Neurol 59:922–928
Jammes Y, Steinberg JG, Mambrini O, Brégeon F, Delliaux S (2005) Chronic fatigue syndrome: assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise. J Intern Med 257:299–310
Lewis SF, Haller RG (1986) The pathophysiology of McArdle’s disease: clues to regulation in exercise and fatigue. J Appl Physiol 61:391–401
Lucia A, Nogales-Gadea G, Perez M, Martin MA, Andreu AL, Arenas J (2008) McArdle disease: what do neurologists need to know? Nat Clin Pract Neurol 4:568–577
Mate-Munoz JL, Moran M, Perez M, Chamorro-Vina C, Gomez-Gallego F, Santiago C, Chicharro L, Foster C, Nogales-Gadea G, Rubio JC, Andreu AL, Martin MA, Arenas J, Lucia A (2007) Favorable responses to acute and chronic exercise in McArdle patients. Clin J Sport Med 17:297–303
McArdle B (1951) Myopathy due to a defect in muscle glycogen breakdown. Clin Sci, pp 13–33
Moritani T, Muro M (1987) Motor unit activity and surface electromyogram power spectrum during increasing force of contraction. Eur J Appl Physiol Occup Physiol 56:260–265
Rubio JC, Garcia-Consuegra I, Nogales-Gadea G, Blazquez A, Cabello A, Lucia A, Andreu AL, Arenas J, Martin MA (2007) A proposed molecular diagnostic flowchart for myophosphorylase deficiency (McArdle disease) in blood samples from Spanish patients. Hum Mutat 28:203–204
Tsujino S, Shanske S, DiMauro S (1993) Molecular genetic heterogeneity of myophosphorylase deficiency (McArdle’s disease). N Engl J Med 329:241–245
Vissing J, Haller RG (2003a) A diagnostic cycle test for McArdle’s disease. Ann Neurol 54:539–542
Vissing J, Haller RG (2003b) The effect of oral sucrose on exercise tolerance in patients with McArdle’s disease. N Engl J Med 349:2503–2509
Vissing J, Vissing SF, MacLean DA, Saltin B, Quistorff B, Haller RG (1998) Sympathetic activation in exercise is not dependent on muscle acidosis. Direct evidence from studies in metabolic myopathies. J Clin Invest 101:1654–1660
Zange J, Grehl T, Disselhorst-Klug C, Rau G, Muller K, Schroder R, Tegenthoff M, Malin JP, Vorgerd M (2003) Breakdown of adenine nucleotide pool in fatiguing skeletal muscle in McArdle’s disease: a noninvasive 31P-MRS and EMG study. Muscle Nerve 27:728–736
Acknowledgments
This work was supported by funding from the South African Medical Research Council, Discovery, the Fondo de Investigaciones Sanitarias (FIS, ref. # PI061183), and the Spanish Ministry of Education (EX-2007-1124).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Fausto Baldissera.
Rights and permissions
About this article
Cite this article
Rae, D.E., Noakes, T.D., San Juan, A.F. et al. Excessive skeletal muscle recruitment during strenuous exercise in McArdle patients. Eur J Appl Physiol 110, 1047–1055 (2010). https://doi.org/10.1007/s00421-010-1585-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1585-5