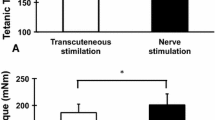
Abstract
The present study investigated the effects of eccentric muscle contractions (ECC) on the content of myofibrillar proteins (my-proteins) and the catalytic activity of myofibrillar ATPase (my-ATPase) in skeletal muscles. Rat extensor digitorum longus and tibialis anterior muscles were exposed to 200-repeated ECC or isometric contractions (ISC) and used for measures of force output and for biochemical analyses, respectively. Whereas in ISC-treated muscles, full restoration of tetanic force was attained after 2 days of recovery, force developed by ECC-treated muscles remained depressed (P < 0.05) after 6 days. The total my-protein content and the relative content of myosin heavy chain (MHC) in total my-proteins were unaltered during 4 days of recovery after ECC, but fell (P < 0.05) to 55.9 and 63.4% after 6 days of recovery, respectively. my-ATPase activity expressed on a my-protein weight basis was unaltered immediately after ECC. However, it decreased (P < 0.05) to 75.3, 45.3, and 49.3% after 2, 4 and 6 days of recovery, respectively. Total maximal calpain activity measured at 5 mM Ca2+ was significantly augmented (P < 0.05) after 2 days of recovery, reaching a level of threefold higher after 6 days. These alterations were specific for ECC and not observed for ISC. These results suggest that depressions in my-ATPase activity contribute to ECC-induced decreases in force and power which can take a number of days to recover.






Similar content being viewed by others
References
Allen DG (2001) Eccentric muscle damage: mechanisms of early reduction of force. Acta Physiol Scand 171:311–319
Baker AJ, Brandes R, Schendel TM, Trocha SD, Miller RG, Weiner MW (1994) Energy use by contractile and noncontractile processes in skeletal muscle estimated by 31P-NMR. Am J Physiol 266:C825–C831
Bárány M (1967) ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol 50:197–218
Bottinelli R, Canepari M, Reggiani C, Stienen GJM (1994) Myofibrillar ATPase activity during isometric contraction and isomyosin composition in rat single skinned muscle fibres. J Physiol (Lond) 481:663–675
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Clarkson PM, Nosaka K, Braun B (1992) Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc 24:512–520
Corona BT, Balog EM, Doyle JA, Rupp JC, Luke RC, Ingalls CP (2010) Junctophilin damage contributes to early strength deficits and EC coupling failure after eccentric contractions. Am J Physiol 298:C365–C376
Dargelos E, Poussard S, Brulé C, Daury L, Cottin P (2008) Calcium-dependent proteolytic system and muscle dysfunctions: a possible role of calpains in sarcopenia. Biochimie 90:359–368
Faulkner JA, Jones DA, Round JM (1989) Injury to skeletal muscles of mice by forced lengthening during contractions. Q J Exp Physiol 74:661–670
Ferreira LF, Reid MB (2008) Muscle-derived ROS and thiol regulation in muscle fatigue. J Appl Physiol 104:853–860
Fielding RA, Manfredi TJ, Ding W, Fiatarone MA, Evans WJ, Cannon JG (1993) Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am J Physiol 265:R166–R172
Fitts RH (2008) The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol 104:551–558
Gagnon J, Gregory P, Kurowski T, Zak R (1990) Changes in protein synthesis during induced muscle fiber transformation. In: Pette D (ed) The dynamic state of muscle fibers. Walter de Gruyter, Berlin, pp 481–495
Gibala MJ, MacDougall JD, Tarnopolsky MA, Stauber WT, Elorriaga A (1995) Changes in human skeletal muscle ultrastructure and force production after acute resistance exercise. J Appl Physiol 78:702–708
Goll DE, Thompson VF, Li H, Wei W, Cong J (2003) The calpain system. Physiol Rev 83:731–801
Ingalls CP, Warren GL, Armstrong RB (1998a) Dissociation of force production from MHC and actin contents in muscles injured by eccentric contractions. J Muscle Res Cell Motil 19:215–224
Ingalls CP, Warren GL, Williams JH, Ward CW, Armstrong RB (1998b) E-C coupling failure in mouse EDL muscle after in vivo eccentric contractions. J Appl Physiol 85:56–67
Ingalls CP, Warren GL, Zhang J-Z, Hamilton SL, Armstrong RB (2004) Dihydropyridine and ryanodine receptor binding after eccentric contraction. J Appl Physiol 96:1619–1625
Keira Y, Noguchi S, Minami N, Hayashi YK, Nishino I (2003) Localization of calpain 3 in human skeletal muscle and its alteration in limb-girdle muscular dystrophy 2A muscle. J Biochem (Tokyo) 133:659–664
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz A-G, Ahn B-W, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Levine RL, Wehr N, Williams JA, Stadtman ER, Shacter E (2000) Determination of carbonyl groups in oxidized proteins. Methods Mol Biol 99:15–24
Lynch GS, Fary CJ, Williams DA (1997) Quantitative measurement of resting skeletal muscle [Ca2+]i following acute and long-term downhill running exercise in mice. Cell Calcium 22:373–383
Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M (2007) FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6:458–471
Matsunaga S, Harmon S, Gohlsch B, Ohlendieck K, Pette D (2001) Inactivation of sarcoplasmic reticulum Ca2+-ATPase in low-frequency stimulated rat muscle. J Muscle Res Cell Motil 22:685–691
Matsunaga S, Inashima S, Yamada T, Watanabe H, Hazama T, Wada M (2003) Oxidation of sarcoplasmic reticulum Ca2+-ATPase induced by high-intensity exercise. Pflügers Arch 446:394–399
Mishima T, Kuratani M, Kanzaki K, Yamada T, Matsunaga S, Wada M (2009) No relationship between enzyme activity and structure of nucleotide binding site in sarcoplasmic reticulum Ca2+-ATPase from short-term stimulated rat muscle. Acta Physiol 196:401–409
Murphy RM, Verburg E, Lamb GD (2006) Ca2+ activation of diffusible and bound pools of μ-calpain in rat skeletal muscle. J Physiol (Lond) 576:595–612
Murphy RM, Goodman CA, McKenna MJ, Bennie J, Leikis M, Lamb GD (2007) Calpain-3 is autolyzed and hence activated in human skeletal muscle 24 h following a single bout of eccentric exercise. J Appl Physiol 103:926–931
Pette D, Staron RS (1990) Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol 116:1–76
Raser KJ, Posner A, Wang KKW (1995) Casein zymography: a method to study micro-calpain, m-calpain, and their inhibitory agents. Arch Biochem Biophys 319:211–216
Rathbone CR, Wenke JC, Warren GL, Armstrong RB (2003) Importance of satellite cells in the strength recovery after eccentric contraction-induced muscle injury. Am J Physiol 285:R1490–R1495
Schollmeyer JE (1986) Role of Ca2+ and Ca2+-activated protease in myoblast fusion. Exp Cell 162:411–422
Sultan KR, Dittrich BT, Pette D (2000) Calpain activity in fast, slow, transforming and regenerating skeletal muscle. Am J Physiol 279:C639–C647
Takekura H, Fujinami N, Nishizawa T, Ogasawara H, Kasuga N (2001) Eccentric exercise-induced morphological changes in the membrane systems involved in excitation-contraction coupling in rat skeletal muscle. J Physiol (Lond) 553:571–583
Talbot JA, Morgan DL (1996) Quantitative analysis of sarcomere non-uniformities in active muscle following a stretch. J Muscle Res Cell Mortil 17:261–268
Termin A, Pette D (1992) Changes in myosin heavy-chain isoform synthesis of chronically stimulated rat fast-twitch muscle. Eur J Biochem 204:569–573
Tsika RW, Herrick RE, Baldwin KM (1987) Interaction of compensatory overload and hindlimb suspension on myosin isoform expression. J Appl Physiol 62:2180–2186
Wada M, Kikuchi K, Katsuta S (1992) Changes in myosin heavy-chain and light-chain isoforms following sustained exercise. In: Sato Y, Poortmans J, Hashimoto I, Oshida Y (eds) Integration of medicine and sports sciences. Karger, Basel, pp 309–317
Yamada T, Mishima T, Sakamoto M, Sugiyama M, Matsunaga S, Wada M (2006) Oxidation of myosin heavy chain and reduction in force production in hyperthyroid rat soleus. J Appl Physiol 100:1520–1526
Yoshida M, Suzuki A, Shimizu T, Ozawa E (1992) Proteinase-sensitive sites on isolated rabbit dystrophin. J Biochem 112:433–439
You T, Goldfarb AH, Bloomer RJ, Nguyen L, Sha X, McKenzie MJ (2005) Oxidative stress response in normal and antioxidant supplemented rats to a downhill run: changes in blood and skeletal muscles. Can J Appl Physiol 30:677–689
Zhang B-T, Yeung SS, Allen DG, Qin L, Yeung EW (2008) Role of the calcium-calpain pathway in cytoskeletal damage after eccentric contractions. J Appl Physiol 105:352–357
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Håkan Westerblad.
Rights and permissions
About this article
Cite this article
Kanzaki, K., Kuratani, M., Mishima, T. et al. The effects of eccentric contraction on myofibrillar proteins in rat skeletal muscle. Eur J Appl Physiol 110, 943–952 (2010). https://doi.org/10.1007/s00421-010-1579-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1579-3