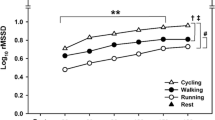
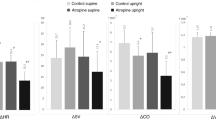
Abstract
It is not known whether subjects that have higher cardiac vagal reactivation (CVR) during repeated exercise transitions also have higher cardiac vagal withdrawal (CVW) at the onset of exercise, which would lead to better heart rate (HR) regulation during exercise transitions. Therefore, our aims were to investigate: (a) the influence of CVR on CVW during repeated rest–exercise transitions; and (b) the influence of the sympathetic activity on CVR and CVW. Fifty-eight healthy men (22 ± 4 years) performed 20 rest–exercise transitions interspaced by 30 s. In addition, nine healthy men (24 ± 3 years) ingested either 25 mg of atenolol or placebo, on a crossover, double-blind, randomized design, then performed 20 rest–exercise transitions interspaced by 30 s. Cardiac vagal reactivation was assessed by a HR variability index (RMSSD) and CVW by the HR increase at the onset of a valid and reliable cycling protocol. The CVR and CVW responses were associated (partial r ranged from 0.60 to 0.66; p < 0.05). Participants with higher CVR over transitions maintained their CVW over repeated transitions [first transition (mean ± SEM) = 1.59 ± 0.04 vs. 20th = 1.50 ± 0.03 (a.u.), p = 0.24], while participants with lower CVR had a CVW decrease over repeated transitions [first transition (mean ± SEM) = 1.38 ± 0.04 vs. 20th = 1.19 ± 0.03 (a.u.), p < 0.01). In addition, the CVR and CVW over the rest–exercise transitions were similar during atenolol and placebo (ANCOVA interaction p = 0.12 and p = 0.48, respectively). In conclusion, the CVR among repeated rest–exercise transitions influenced the CVW at the onset of exercise, which was not affected by a partial β1 cardioselective adrenoceptor blockade.




Similar content being viewed by others
References
Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ (1981) Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 213:220–222
Araújo CG, Nóbrega AC, Castro CL (1992) Heart rate responses to deep breathing and 4-seconds of exercise before and after pharmacological blockade with atropine and propranolol. Clin Auton Res 2:35–40
Araújo CGS, Ricardo DR, Almeida MB (2003) Intra and interdays reliability of the 4-second exercise test. Braz J Sports Med 9:299–303
Bangsbo J (1994) The physiology of soccer—with special reference to intense intermittent exercise. Acta Physiol Scand Suppl 619:1–155
Brown HC, Carruthers SG, Johnston GD, Kelly JG, McAinsh J, McDevitt DG, Shanks RG (1976) Clinical pharmacologic observations on atenolol, a beta-adrenoceptor blocker. Clin Pharmacol Ther 20:524–534
Buchheit M, Laursen PB, Ahmaidi S (2007) Parasympathetic reactivation after repeated sprint exercise. Am J Physiol Heart Circ Physiol 293:H133–H141
Castro CL, Nóbrega AC, Araújo CG (1992a) Autonomic cardiovascular tests. A critical review I. Arq Bras Cardiol 59:75–85
Castro CL, Nóbrega AC, Araújo CG (1992b) Autonomic cardiovascular tests. A critical review II. Arq Bras Cardiol 59:151–158
Cooke WH, Cox JF, Diedrich AM, Taylor JA, Beightol LA, Ames JE 4th, Hoag JB, Seidel H, Eckberg DL (1998) Controlled breathing protocols probe human autonomic cardiovascular rhythms. Am J Physiol 274:H709–H718
Cruickshank JM, Neil-Dwyer G (1985) Beta-blocker brain concentrations in man. Eur J Clin Pharmacol 28 Suppl: 21-23
DiCarlo SE, Bishop VS (1992) Onset of exercise shifts operating point of arterial baroreflex to higher pressures. Am J Physiol 262:H303–H307
Dupont G, Millet GP, Guinhouya C, Berthoin S (2005) Relationship between oxygen uptake kinetics and performance in repeated running sprints. Eur J Appl Physiol 95:27–34
Fagraeus L, Linnarsson D (1976) Autonomic origin of heart rate fluctuations at the onset of muscular exercise. J Appl Physiol 40:679–682
Fritzsche RG, Switzer TW, Hodgkinson BJ, Coyle EF (1999) Stroke volume decline during prolonged exercise is influenced by the increase in heart rate. J Appl Physiol 86:799–805
Goldberger JJ, Le FK, Lahiri M, Kannankeril PJ, Ng J, Kadish AH (2006) Assessment of parasympathetic reactivation after exercise. Am J Physiol Heart Circ Physiol 290:H2446–H2452
Hashimoto I, Miyamura M, Saito M (1998) Initiation of increase in muscle sympathetic nerve activity delay during maximal voluntary contraction. Acta Physiol Scand 164:293–297
Javorka M, Zila I, Balharek T, Javorka K (2003) On- and off-responses of heart rate to exercise—relations to heart rate variability. Clin Physiol Funct Imaging 23:1–8
Lador F, Azabji Kenfack M, Moia C, Cautero M, Morel DR, Capelli C, Ferretti G (2006) Simultaneous determination of the kinetics of cardiac output, systemic O(2) delivery, and lung O(2) uptake at exercise onset in men. Am J Physiol Regul Integr Comp Physiol 290:R1071–R1079
Leeper NJ, Dewey FE, Ashley EA, Sandri M, Tan SY, Hadley D, Myers J, Froelicher V (2007) Prognostic value of heart rate increase at onset of exercise testing. Circulation 115:468–474
Martin PJ, Levy JR, Wexberg S, Levy MN (1983) Phasic effects of repetitive vagal stimulation on atrial contraction. Circ Res 52:657–663
McCloskey DI, Mitchell JH (1972) Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224:173–186
McMahon SE, McWilliam PN (1992) Changes in R–R interval at the start of muscle contraction in the decerebrate cat. J Physiol 447:549–562
Nóbrega AC, Araújo CG (1993) Heart rate transient at the onset of active and passive dynamic exercise. Med Sci Sports Exerc 25:37–41
Nóbrega AC, Castro CL, Araújo CG (1990) Relative roles of the sympathetic and parasympathetic systems in the 4-s exercise test. Braz J Med Biol Res 23:1259–1262
Oliveira RB, Vianna LC, Ricardo DR, de Almeida MB, Araújo CG (2006) Influence of different respiratory maneuvers on exercise-induced cardiac vagal inhibition. Eur J Appl Physiol 97:607–612
Parker GW, Michael LH, Hartley CJ, Skinner JE, Entman ML (1990) Central beta-adrenergic mechanisms may modulate ischemic ventricular fibrillation in pigs. Circ Res 66:259–270
Perini R, Orizio C, Comande A, Castellano M, Beschi M, Veicsteinas A (1989) Plasma norepinephrine and heart rate dynamics during recovery from submaximal exercise in man. Eur J Appl Physiol Occup Physiol 58:879–883
Potter EK (1985) Prolonged non-adrenergic inhibition of cardiac vagal action following sympathetic stimulation: neuromodulation by neuropeptide Y? Neurosci Lett 54:117–121
Ramos PS, Araújo CG (2010) Lower cardiac vagal tone in non-obese healthy men with unfavorable anthropometric characteristics. Clinics 65:45–51
Ricardo DR, de Almeida MB, Franklin BA, Araújo CG (2005) Initial and final exercise heart rate transients: influence of gender, aerobic fitness, and clinical status. Chest 127:318–327
Sandercock GR, Bromley PD, Brodie DA (2005) Effects of exercise on heart rate variability: inferences from meta-analysis. Med Sci Sports Exerc 37:433–439
Silva BM, Vianna LC, Oliveira RB, Ricardo DR, Araújo CG (2008) Similar cardiac vagal withdrawal at the onset of arm and leg dynamic exercise. Eur J Appl Physiol 102:695–701
Uusitalo AL, Vanninen E, Levalahti E, Battie MC, Videman T, Kaprio J (2007) Role of genetic and environmental influences on heart rate variability in middle-aged men. Am J Physiol Heart Circ Physiol 293:H1013–H1022
Vaile JC, Fletcher J, Al-Ani M, Ross HF, Littler WA, Coote JH, Townend JN (1999) Use of opposing reflex stimuli and heart rate variability to examine the effects of lipophilic and hydrophilic beta-blockers on human cardiac vagal control. Clin Sci (Lond) 97:585–593 (discussion 609–510)
Vianna LC, Oliveira RB, Ramos PS, Ricardo DR, Araújo CG (2010) Effect of muscle mass on muscle mechanoreflex-mediated heart rate increase at the onset of dynamic exercise. Eur J Appl Physiol 108:429–434
Wieling W, Harms MP, ten Harkel AD, van Lieshout JJ, Sprangers RL (1996) Circulatory response evoked by a 3 s bout of dynamic leg exercise in humans. J Physiol 494:601–611
Williamson JW, Nóbrega AC, Winchester PK, Zim S, Mitchell JH (1995) Instantaneous heart rate increase with dynamic exercise: central command and muscle-heart reflex contributions. J Appl Physiol 78:1273–1279
Williamson JW, Fadel PJ, Mitchell JH (2006) New insights into central cardiovascular control during exercise in humans: a central command update. Exp Physiol 91:51–58
Acknowledgments
The authors are grateful to all the students and university staff from SUPREMA that collaborated to accomplish the study. Bruno Moreira Silva and Lauro Casqueiro Vianna were supported by scholarships from the National Council of Scientific and Technologic Development, CNPq, Brazil and Dr. Claudio Gil S. Araújo received a grant from CNPq for scientific production
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dag Linnarsson.
Rights and permissions
About this article
Cite this article
Ricardo, D.R., Silva, B.M., Vianna, L.C. et al. Cardiac vagal withdrawal and reactivation during repeated rest–exercise transitions. Eur J Appl Physiol 110, 933–942 (2010). https://doi.org/10.1007/s00421-010-1555-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1555-y