Abstract
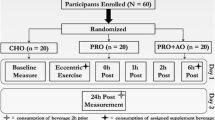
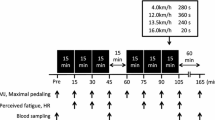
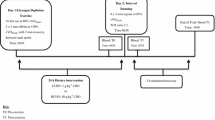
Inflammation associated with adipose tissue is modulated by macronutrient availability. For example, glucose increases inflammation in obese but not lean individuals. Little is known about how macronutrient intake influences inflammation associated with muscle. The aim of this study was to determine the impact of macronutrient intake differences during recovery from eccentric exercise on the inflammatory response. The study was a cross-over design in which young men and women (n = 12) completed high and low carbohydrate (CHO) conditions. Both conditions consisted of six sets of ten maximal high-force eccentric contractions of the elbow flexors and extensors followed by a controlled diet for the first 8 h post-exercise. Glucose, insulin, tumor necrosis factor-α, interleukin (IL)-1β, IL-6, and C-reactive protein were measured from blood samples pre-exercise, 1.5, 4, 8, and 24 h post-exercise. Perceived muscle soreness, strength loss, and serum CK activity were measured through 120 h post-exercise. Perceived soreness was elevated (P < 0.001) at all time points post-exercise in both conditions and was higher (P < 0.05) in the high compared to the low CHO condition. IL-1β increased (P = 0.05) 24 h post-exercise in the high compared to the low CHO condition. There was a trend (P = 0.06) for IL-6 to be elevated in the high compared to the low CHO condition. We conclude that inflammation induced by high-force eccentric exercise in skeletal muscle is greater when a high CHO compared to a low CHO diet is consumed during recovery.





Similar content being viewed by others
References
Aderka D, Sorkine P, Abu-Abid S, Lev D, Setton A, Cope AP, Wallach D, Klausner J (1998) Shedding kinetics of soluble tumor necrosis factor (TNF) receptors after systemic TNF leaking during isolated limb perfusion. Relevance to the pathophysiology of septic shock. J Clin Invest 101:650–659
Beutler BA, Milsark IW, Cerami A (1985) Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol 135:3972–3977
Bruunsgaard H, Galbo H, Halkjaer-Kristensen J, Johansen TL, MacLean DA, Pedersen BK (1997) Exercise-induced increase in serum interleukin-6 in humans is related to muscle damage. J Physiol 499(Pt 3):833–841
Dandona P, Chaudhuri A, Ghanim H, Mohanty P (2007) Proinflammatory effects of glucose and anti-inflammatory effect of insulin: relevance to cardiovascular disease. Am J Cardiol 99:15B–26B
Davis JM, Murphy EA, Carmichael MD, Zielinski MR, Groschwitz CM, Brown AS, Gangemi JD, Ghaffar A, Mayer EP (2007) Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol 292:R2168–R2173
Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D (2002) Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 106:2067–2072
Faulkner JA, Brooks SV, Opiteck JA (1993) Injury to skeletal muscle fibers during contractions: conditions of occurrence and prevention. Phys Ther 73:911–921
Flores EA, Bistrian BR, Pomposelli JJ, Dinarello CA, Blackburn GL, Istfan NW (1989) Infusion of tumor necrosis factor/cachectin promotes muscle catabolism in the rat. A synergistic effect with interleukin 1. J Clin Invest 83:1614–1622
Foster-Powell K, Holt SH, Brand-Miller JC (2002) International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 76:5–56
Gonzalez F, Minium J, Rote NS, Kirwan JP (2006) Altered tumor necrosis factor alpha release from mononuclear cells of obese reproductive-age women during hyperglycemia. Metabolism 55:271–276
Grundy SM, Cleeman JI, Daniels SR et al (2005) Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–2752
Hirose L, Nosaka K, Newton M, Laveder A, Kano M, Peake J, Suzuki K (2004) Changes in inflammatory mediators following eccentric exercise of the elbow flexors. Exerc Immunol Rev 10:75–90
Ivy JL, Ding Z, Hwang H, Cialdella-Kam LC, Morrison PJ (2008) Post exercise carbohydrate-protein supplementation: phosphorylation of muscle proteins involved in glycogen synthesis and protein translation. Amino Acids 35:89–97
Loram LC, Fuller A, Fick LG, Cartmell T, Poole S, Mitchell D (2007) Cytokine profiles during carrageenan-induced inflammatory hyperalgesia in rat muscle and hind paw. J Pain 8:127–136
Manninen AH (2006) Hyperinsulinaemia, hyperaminoacidaemia and post-exercise muscle anabolism: the search for the optimal recovery drink. Br J Sports Med 40:900–905
Miles MP, Walker EE, Conant SB, Hogan SP, Kidd JR (2006) Carbohydrate influences plasma interleukin-6 but not C-reactive protein or creatine kinase following a 32-km mountain trail race. Int J Sport Nutr Exerc Metab 17:36–46
Miles MP, Pearson SD, Andring JM, Kidd JR, Volpe SL (2007) Effect of carbohydrate intake during recovery from eccentric exercise on interleukin-6 and muscle-damage markers. Int J Sport Nutr Exerc Metab 17:507–520
Miles MP, Andring JM, Pearson SD, Gordon LK, Kasper C, Depner CM, Kidd JR (2008) Diurnal variation, response to eccentric exercise, and association of inflammatory mediators with muscle damage variables. J Appl Physiol 104:451–458
Moldawer LL, Svaninger G, Gelin J, Lundholm KG (1987) Interleukin 1 and tumor necrosis factor do not regulate protein balance in skeletal muscle. Am J Physiol 253:C766–C773
Nawabi MD, Block KP, Chakrabarti MC, Buse MG (1990) Administration of endotoxin, tumor necrosis factor, or interleukin 1 to rats activates skeletal muscle branched-chain alpha-keto acid dehydrogenase. J Clin Invest 85:256–263
Negro M, Giardina S, Marzani B, Marzatico F (2008) Branched-chain amino acid supplementation does not enhance athletic performance but affects muscle recovery and the immune system. J Sports Med Phys Fitness 48:347–351
Nehlsen-Cannarella SL, Fagoaga OR, Nieman DC, Henson DA, Butterworth DE, Schmitt RL, Bailey EM, Warren BJ, Utter A, Davis JM (1997) Carbohydrate and the cytokine response to 2.5 h of running. J Appl Physiol 82:1662–1667
Nieman DC, Davis JM, Brown VA, Henson DA, Dumke CL, Utter AC, Vinci DM, Downs MF, Smith JC, Carson J, Brown A, McAnulty SR, McAnulty LS (2004) Influence of carbohydrate ingestion on immune changes after 2 h of intensive resistance training. J Appl Physiol 96:1292–1298
Nosaka K, Clarkson PM, McGuiggin ME, Byrne JM (1991) Time course of muscle adaptation after high force eccentric exercise. Eur J Appl Physiol Occup Physiol 63:70–76
Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK (1999) Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol 515(Pt 1):287–291
Paulsen G, Benestad HB, Strom-Gundersen I, Morkrid L, Lappegard KT, Raastad T (2005) Delayed leukocytosis and cytokine response to high-force eccentric exercise. Med Sci Sports Exerc 37:1877–1883
Pearson TA, Mensah GA, Alexander RW et al (2003) Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107:499–511
Pedersen BK, Ostrowski K, Rohde T, Bruunsgaard H (1998) The cytokine response to strenuous exercise. Can J Physiol Pharmacol 76:505–511
Petersen AM, Pedersen BK (2005) The anti-inflammatory effect of exercise. J Appl Physiol 98:1154–1162
Plomgaard P, Bouzakri K, Krogh-Madsen R et al (2005) Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 54:2939–2945
Proske U, Morgan DL (2001) Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol 537:333–345
Silva LA, Silveira PC, Pinho CA, Tuon T, Dal Pizzol F, Pinho RA (2008) N-acetylcysteine supplementation and oxidative damage and inflammatory response after eccentric exercise. Int J Sport Nutr Exerc Metab 18:379–388
Smith LL, Miles MP (2000) Exercise-induced muscle injury and inflammation. In: Garrett WE, Kirkendall DT (eds) Exercise and sport science. Lippincott Williams & Wilkins, Philadelphia, pp 401–411
Smith LL, Anwar A, Fragen M, Rananto C, Johnson R, Holbert D (2000) Cytokines and cell adhesion molecules associated with high-intensity eccentric exercise. Eur J Appl Physiol 82:61–67
Tidball JG (2005) Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288:R345–R353
Tilg H, Moschen AR (2008) Inflammatory mechanisms in the regulation of insulin resistance. Mol Med 14:222–231
Acknowledgments
This study was funded by the Department of Health and Human Development at Montana State University and an ADVANCE Leadership grant from the National Science Foundation.
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by William Kraemer.
Rights and permissions
About this article
Cite this article
Depner, C.M., Kirwan, R.D., Frederickson, S.J. et al. Enhanced inflammation with high carbohydrate intake during recovery from eccentric exercise. Eur J Appl Physiol 109, 1067–1076 (2010). https://doi.org/10.1007/s00421-010-1448-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1448-0