Abstract
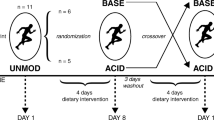
This study examined the effects of NH4Cl ingestion on phosphocreatine (PCr) metabolism during 9 min of moderate- (MOD) and heavy- (HVY) intensity constant-load isotonic plantar-flexion exercise. Healthy young adult male subjects (n = 8) completed both a control (CON) and NH4Cl ingestion (ACID) trial. Phosphorus-31 magnetic resonance spectroscopy was used to monitor changes in intracellular pH (pHi), [Pi], [PCr], and [ATP]. During the Middle (3–6 min) and Late (6–9 min) stages of HVY, ACID was associated with a higher (P < 0.05) intracellular hydrogen-ion concentration ([H+]i) [Middle: 246 (SD 36) vs. 202 (SD 36) mmol/l]; [Late: 236 (SD 35) vs. 200 (SD 39) mmol/l]. In addition, ACID was associated with a lower (P < 0.05) [PCr] relative to CON during the Early (0–3 min) [18.1 (SD 5.1) vs. 20.4 (SD 5.4) mmol/l] and Middle stages [14.1 (SD 5.4) vs. 16.7 (SD 6.0) mmol/l] of HVY. The amplitude of the primary component of PCr breakdown during the transition to HVY was greater in ACID than CON [14.5 (SD 5.8 vs. 11.3 (SD 4.8) mmol/l], however, the PCr slow component (continued slow decline in [PCr]) showed no difference (P > 0.05). The time constant for PCr breakdown (τPCr) was greater in HVY than MOD for both conditions [58 (SD 22) vs. 28 (SD 15) s ACID; 51 (SD 20) vs. 29 (SD 14) s CON] (P < 0.05). In summary, ACID increased PCr breakdown during the transition from MOD to HVY, but did not increase the magnitude of the PCr slow component.







Similar content being viewed by others
References
Allen DG, Lamb GD, Westerblad H (2008) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88:287–332
Barstow TJ, Buchthal S, Zanconato S, Cooper DM (1994) Muscle energetics and pulmonary oxygen uptake kinetics during moderate exercise. J Appl Physiol 77:1742–1749
Barstow TJ, Jones AM, Nguyen PH, Casaburi R (1996) Influence of muscle fibre and pedal frequency on oxygen uptake kinetics of heavy exercise. J Appl Physiol 81:1642–1650
Bartha R, Drost DJ, Williamson PC (1999) Factors affecting the quantification of short echo in-vivo 1H MR spectra: prior knowledge, peak elimination, and filtering. NMR Biomed 12:205–216
Cannon DT, Kolkhorst FW, Cipriani DJ (2007) Electromyographic data do not support a progressive recruitment of muscle fibres during exercise exhibiting a VO2 slow component. J Physiol Anthropol 26:541–546
Chance B, Conrad H (1959) Acid-linked functions of intermediates in oxidative phosphorylation. II. Experimental studies of the effect of pH upon respiratory, phosphorylative and transfer activities of liver and heart mitochondria. J Biol Chem 234:1568–1570
Chance B, Williams GR (1955) Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem 217:383–393
Chance B, Williams CM (1956) The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem 17:65–134
Chance B, Leigh JS, Clark BJ, Maris J, Kent J, Nioka S, Smith D (1985) Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proc Natl Acad Sci USA 82:8384–8388
Conley KE, Kemper WF, Crowther GJ (2001) Limits to sustainable muscle performance: interaction between glycolysis and oxidative phosphorylation. J Exp Biol 204:3189–3194
Crow MT, Kushmerick MJ (1982) Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol 79:147–166
Fitts RH (1994) Cellular mechanisms of muscle fatigue. Physiol Rev 74:49–94
Forbes SC, Raymer GH, Kowalchuk JM, Marsh GD (2005) NaHCO3 −-induced alkalosis reduces the phosphocreatine slow component during heavy-intensity forearm exercise. J Appl Physiol 99:1668–1675
Forbes SC, Kowalchuk JM, Thompson RT, Marsh GD (2007) Effects of hyperventilation on phosphocreatine kinetics and muscle deoxygenation during moderate-intensity plantar flexion exercise. J Appl Physiol 102:1565–1573
Forbes SC, Raymer GH, Kowalchuk JM, Thompson RT, Marsh GD (2008) Effects of recovery time on phosphocreatine kinetics during repeated bouts of heavy intensity exercise. Eur J Appl Physiol 103:665–675
Garland SW, Wang W, Ward SA (2006) Indices of electromyographic activity and the “slow” component of oxygen uptake kinetics during high-intensity knee-extension exercise in humans. Eur J Appl Physiol 97:413–423
Golding EM, Teague WE, Dobson GP (1995) Adjustment of K′ to varying pH and pMg for the creatine kinase, adenylate kinase and ATP hydrolysis equilibria permitting quantitative bioenergetic assessment. J Exp Biol 198:1775–1782
Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD (1996) Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol 80:988–998
Harkema SJ, Meyer RA (1997) Effect of acidosis on control of respiration in skeletal muscle. Am J Physiol 272:C491–C500
Harkema SJ, Adams GR, Meyer RA (1997) Acidosis has no effect on the ATP cost of contraction in cat fast- and slow-twitch skeletal muscles. Am J Physiol 272:C485–C490
Harris RC, Hultman E, Nordesjo LO (1974) Glycogen, glycolytic intermediates and high energy phosphates determined in biopsy samples of musculus quadriceps of man at rest: methods and variance of values. Scand J Clin Lab Invest 33:109–120
Hasler LJ, Kindig CA, Richardson RS, Hogan MC (2004) The role of oxygen in determining phosphocreatine onset kinetics in exercising humans. J Physiol 558:985–992
Hollidge-Horvat MG, Parolin ML, Wong D, Jones NL, Heigenhauser GJF (1999) Effect of induced metabolic acidosis on human skeletal muscle metabolism during exercise. Am J Physiol 277:E647–E658
Hood VL, Schubert C, Keller U, Muller S (1988) Effect of systemic pH and pHi and lactic acid generation in exhaustive forearm exercise. Am J Physiol 255:F479–F485
Hughson RL, Morrissey M (1982) Delayed kinetics of respiratory gas exchange in the transition from prior exercise. J Appl Physiol 52:921–929
Hughson RL, Morrissey M (1983) Delayed kinetics of O2 in the transition from prior exercise. Evidence for O2 transport limitation of O2 kinetics: a review. Int J Sports Med 4:31–39
Hultman E, Del Canale S, Sjoholm H (1985) Effect of induced metabolic acidosis on intracellular pH, buffer capacity and contraction force of human skeletal muscle. Clin Sci 69:505–510
Jones NL, Sutton JR, Taylor R, Toews CJ (1977) Effect of pH on cardiorespiratory and metabolic responses to exercise. J Appl Physiol 43:959–964
Jones AM, Wilkerson DP, Fulford J (2008) Muscle [phosphocreatine] dynamics following the onset of exercise in humans: the influence of baseline work-rate. J Physiol 586:889–898
Jubrais SA, Crowther GJ, Shankland EG, Gronka RK, Conley KE (2003) Acidosis inhibits oxidative phosphorylation in contracting human skeletal muscle in vivo. J Physiol 553:589–599
Kowalchuk JM, Heigenhauser GJF, Jones NL (1984) Effect of pH on metabolic and cardiorespiratory responses during progressive exercise. J Appl Physiol 57:1558–1563
Kushmerick MJ, Meyer RA, Brown TR (1992) Regulation of oxygen consumption in fast- and slow-twitch muscle. Am J Physiol 263:C598–C606
MacPhee SL, Shoemaker JK, Paterson DH, Kowalchuk JM (2005) Kinetics of O2 uptake, leg blood flow, and muscle deoxygenation are slowed in the upper compared with lower region of the moderate-intensity exercise domain. J Appl Physiol 99:1822–1834
Mahler M (1985) First-order kinetics of muscle oxygen consumption, and an equivalent proportionality between QO2 and phosphorylcreatine level. Implications for the control of respiration. J Gen Physiol 86:135–165
Marquardt DW (1963) An algorithm for least-squares estimation of non-linear parameters. J Soc Ind Appl Math 11:431–441
Marsh GD, Paterson DH, Potwarka JJ, Thompson RT, Driedger AA (1991) Coincident thresholds in intracellular phosphorylation potential and pH during progressive exercise. J Appl Physiol 71:1076–1081
Marsh GD, Paterson DH, Potwarka JJ, Thompson RT (1993) Transient changes in muscle high-energy phosphates during moderate exercise. J Appl Physiol 75:648–656
McCreary CR, Chilibeck PD, Marsh GD, Paterson DH, Cunningham DA, Thompson RT (1996) Kinetics of pulmonary oxygen uptake and muscle phosphates during moderate-intensity calf exercise. J Appl Physiol 81:1331–1338
Meyer RA (1988) A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol 254:C548–C553
Meyer RA (1989) Linear dependence of muscle phosphocreatine kinetics on total creatine content. Am J Physiol 257:C1149–C1157
Poole DC, Schaffartzik W, Knight DR, Derion T, Kennedy B, Guy HJ, Prediletto R, Wagner PD (1991) Contribution of the exercising legs to the slow component of oxygen uptake kinetics in humans. J Appl Physiol 71:1245–1260
Poole DC, Barstow TJ, Gaesser GA, Willis WT, Whipp BJ (1994) VO2 slow component: physiological and functional significance. Med Sci Sports Exerc 26:1354–1358
Raymer GH, Allman BL, Rice CL, Marsh GD, Thompson RT (2006) Characteristics of an MR-compatible ankle exercise ergometer for a 3.0 T head-only MR scanner. Med Eng Phys 28:489–494
Rossiter HB, Ward SA, Doyle VA, Howe FA, Griffiths JR, Whipp BJ (1999) Inferences from pulmonary O2 uptake with respect to intramuscular [phosphocreatine] kinetics during moderate exercise in humans. J Physiol 518:921–932
Rossiter HB, Ward SA, Howe FA, Kowalchuk JM, Griffiths JR, Whipp BJ (2002a) Dynamics of intramuscular 31P-MRS Pi peak splitting and the slow components of PCr and O2 uptake during exercise. J Appl Physiol 93:2059–2069
Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ (2002b) Dynamic asymmetry of phosphocreatine concentration and O2 uptake between the on- and off-transients of moderate- and high-intensity exercise in humans. J Physiol 541:991–1002
Rossiter HB, Ward SA, Howe FA, Wood DM, Kowalchuk JM, Griffiths JR, Whipp BJ (2003) Effects of dichloroacetate on VO2 and intramuscular 31P metabolite kinetics during high-intensity exercise in humans. J Appl Physiol 95:1105–1115
Sahlin K, Edstrom L, Sjoholm H (1983) Fatigue and phosphocreatine depletion during carbon dioxide-induced acidosis in rat muscle. Am J Physiol 245:C15–C20
Santalla A, Perez M, Montilla M, Vicente L, Davison R, Earnest C, Lucia A (2003) Sodium bicarbonate ingestion does not alter the slow component of oxygen uptake kinetics in professional cyclists. J Sports Sci 21:39–47
Scheuermann BW, Hoelting BD, Noble ML, Barstow TJ (2001) The slow component of O2 uptake is not accompanied by changes in muscle EMG during repeated bouts of heavy exercise in humans. J Physiol 15:245–256
Taylor DJ, Bore PJ, Styles P, Gadian DG, Radda GK (1983) Bioenergetics of intact human muscle: a 31P nuclear magnetic resonance study. Mol Biol Med 1:77–94
Tordi N, Perrey S, Harvey A, Hughson RL (2003) Oxygen uptake kinetics during two bouts of heavy cycling separated by fatiguing sprint exercise in humans. J Appl Physiol 94:533–541
Veith E (1989) Fitting piecewise linear regression functions to biological responses. J Appl Physiol 67:390–396
Walsh B, Tiivel T, Tonkonogi M, Sahlin K (2002) Increased concentrations of Pi and lactic acid reduce creatine-stimulated respiration in muscle fibres. J Appl Physiol 92:2273–2276
Whipp BJ, Wasserman K (1972) Oxygen uptake kinetics for various intensities of constant-load work. J Appl Physiol 33:351–356
Whipp BJ, Rossiter HB, Ward SA (2002) Exertional oxygen uptake kinetics: a stamen of stamina? Biochem Soc Trans 30:237–247
Wu F, Yang F, Vinnakota KC, Beard DA (2007) Computer modeling of mitochondrial tricarboxylic acid cycle, oxidative phosphorylation, metabolite transport, and electrophysiology. J Biol Chem 282:24525–24537
Zoladz JA, Duda K, Majerczak J, Domanski J, Emmerich J (1997) Metabolic alkalosis induced by pre-exercise ingestion of NaHCO3 does not modulate the slow component of VO2 kinetics in humans. J Physiol Pharmacol 48:211–223
Zoladz JA, Duda K, Majerczak J, Emmerich J, Domanski J (1998) Pre-exercise acidification induced by ingestion of NH4Cl increases the magnitude of the slow component of VO2 kinetics in humans. J Physiol Pharmacol 49:443–455
Zoladz JA, Gladden LB, Hogan MC, Nieckarz Z, Grassi B (2008) Progressive recruitment of muscle fibres is not necessary for the slow component of VO2 kinetics. J Appl Physiol 105:575–580
Acknowledgments
We thank Dr. R. T. Thompson in the Imaging Division of the Lawson Health Research Institute for his technical assistance and for providing time on the 3T. We thank the study participants for their compliance with the protocol.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Susan Ward.
Rights and permissions
About this article
Cite this article
Churchward-Venne, T.A., Kowalchuk, J.M. & Marsh, G.D. Effects of ammonium chloride ingestion on phosphocreatine metabolism during moderate- and heavy-intensity plantar-flexion exercise. Eur J Appl Physiol 108, 1189–1200 (2010). https://doi.org/10.1007/s00421-009-1327-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-009-1327-8