Abstract
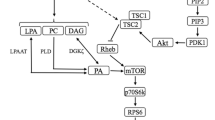
Resistance exercise disturbs skeletal muscle homeostasis leading to activation of catabolic and anabolic processes within the muscle cell. A current challenge of exercise biology is to describe the molecular mechanisms of regulation by which contractile activity stimulates net protein breakdown during exercise and net protein synthesis during recovery. Muscle growth is optimized by combining exercise and appropriate nutritional strategies, such as amino acid (AA) and carbohydrate ingestion. The effects are integrated at the level of one central regulatory protein, mTOR (mammalian target of rapamycin). mTOR is a complex protein integrating signals of the energetic status of the cell and environmental stimuli to control protein synthesis, protein breakdown and therefore cell growth. mTOR is known to be activated by insulin, and the mechanisms involved are well documented. The ways by which exercise and AA lead to mTOR activation remain partially unclear. Exercise and AA use different signalling pathways upstream of mTOR. Exercise seems to recruit partially the same pathway as insulin, whereas AA could act more directly on mTOR. During resistance exercise, the activity of mTOR could be acutely blunted by AMP-activated protein kinase (AMPK), thus inhibiting protein synthesis and enhancing AA availability for energy metabolism. During recovery, the inhibition of mTOR by AMPK is suppressed, and its activation is maximized by the presence of AA. There appears to be a requirement for a minimal concentration of plasma insulin to stimulate muscle protein synthesis in response to resistance exercise and AA ingestion.




Similar content being viewed by others
References
Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR (2000) Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 130:2413–2419
Baar K, Esser K (1999) Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol 276:C120–C127
Backer JM, Myers MG Jr, Sun XJ, Chin DJ, Shoelson SE, Miralpeix M, White MF (1993) Association of IRS-1 with the insulin receptor and the phosphatidylinositol 3′-kinase. Formation of binary and ternary signaling complexes in intact cells. J Biol Chem 268:8204–8212
Berven LA, Crouch MF (2000) Cellular function of p70S6K: a role in regulating cell motility. Immunol Cell Biol 78:447–451
Berven LA, Willard FS, Crouch MF (2004) Role of the p70(S6K) pathway in regulating the actin cytoskeleton and cell migration. Exp Cell Res 296:183–195
Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR (1995) Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol 268:E514–E520
Biolo G, Tipton KD, Klein S, Wolfe RR (1997) An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol 273:E122–E129
Blommaart EF, Luiken JJ, Blommaart PJ, van Woerkom GM, Meijer AJ (1995) Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem 270:2320–2326
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019
Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, Kimball SR, Jefferson LS (2003) Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol (Lond) 553:213–220
Browne GJ, Proud CG (2002) Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem 269:5360–5368
Browne GJ, Finn SG, Proud CG (2004) Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem 279:12220–12231
Brozinick JT Jr, Birnbaum MJ (1998) Insulin, but not contraction, activates Akt/PKB in isolated rat skeletal muscle. J Biol Chem 273:14679–14682
Brunn GJ, Fadden P, Haystead TA, Lawrence JC Jr (1997) The mammalian target of rapamycin phosphorylates sites having a (Ser/Thr)-Pro motif and is activated by antibodies to a region near its COOH terminus. J Biol Chem 272:32547–32550
Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM (1998) RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci U S A 95:1432–1437
Chen ZP, Stephens TJ, Murthy S, Canny BJ, Hargreaves M, Witters LA, Kemp BE, McConell GK (2003) Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes 52:2205–2212
Cheng SW, Fryer LG, Carling D, Shepherd PR (2004) Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J Biol Chem 279:15719–15722
Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR (1997) The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem 272:6653–6662
Cooper CE, Vollaard NB, Choueiri T, Wilson MT (2002) Exercise, free radicals and oxidative stress. Biochem Soc Trans 30:280–285
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785–789
Dennis PB, Fumagalli S, Thomas G (1999) Target of rapamycin (TOR): balancing the opposing forces of protein synthesis and degradation. Curr Opin Genet Dev 9:49–54
Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G (2001) Mammalian TOR: a homeostatic ATP sensor. Science 294:1102–1105
Farrell PA, Fedele MJ, Vary TC, Kimball SR, Lang CH, Jefferson LS (1999) Regulation of protein synthesis after acute resistance exercise in diabetic rats. Am J Physiol 276:E721–E727
Gao N, Flynn DC, Zhang Z, Zhong XS, Walker V, Liu KJ, Shi X, Jiang BH (2004) G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am J Physiol 287:C281–C291
Gao X, Zhang Y, Arrazola P, Hino O, Kobayashi T, Yeung RS, Ru B, Pan D (2002) Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat Cell Biol 4:699–704
Goldspink G (1999) Changes in muscle mass and phenotype and the expression of autocrine and systemic growth factors by muscle in response to stretch and overload. J Anat 194(3):323–334
Hernandez JM, Fedele MJ, Farrell PA (2000) Time course evaluation of protein synthesis and glucose uptake after acute resistance exercise in rats. J Appl Physiol 88:1142–1149
Im E, von Lintig FC, Chen J, Zhuang S, Qui W, Chowdhury S, Worley PF, Boss GR, Pilz RB (2002) Rheb is in a high activation state and inhibits B-Raf kinase in mammalian cells. Oncogene 21:6356–6365
Inoki K, Li Y, Zhu T, Wu J, Guan KL (2002) TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4:648–657
Inoki K, Li Y, Xu T, Guan KL (2003a) Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev 17:1829–1834
Inoki K, Zhu T, Guan KL (2003b) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115:577–590
Jefferson LS, Kimball SR (2003) Amino acids as regulators of gene expression at the level of mRNA translation. J Nutr 133:2046S–2051S
Ji G, Barsotti RJ, Feldman ME, Kotlikoff MI (2002) Stretch-induced calcium release in smooth muscle. J Gen Physiol 119:533–544
Kamada Y, Funakoshi T, Shintani T, Nagano K, Ohsumi M, Ohsumi Y (2000) Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J Cell Biol 150:1507–1513
Kanazawa T, Taneike I, Akaishi R, Yoshizawa F, Furuya N, Fujimura S, Kadowaki M (2004) Amino acids and insulin control autophagic proteolysis through different signaling pathways in relation to mTOR in isolated rat hepatocytes. J Biol Chem 279:8452–8459
Karlsson HK, Nilsson PA, Nilsson J, Chibalin AV, Zierath JR, Blomstrand E (2004) Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise. Am J Physiol 287:E1–7
Kasuga M, Karlsson FA, Kahn CR (1982) Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science 215:185–187
Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA (1999) Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem Sci 24:22–25
Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110:163–175
Kimball SR, Horetsky RL, Jefferson LS (1998) Implication of eIF2B rather than eIF4E in the regulation of global protein synthesis by amino acids in L6 myoblasts. J Biol Chem 273:30945–30953
Kimball SR, Farrell PA, Jefferson LS (2002) Invited review: Role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J Appl Physiol 93:1168–1180
Li Y, Inoki K, Yeung R, Guan KL (2002) Regulation of TSC2 by 14-3-3 binding. J Biol Chem 277:44593–44596
Li Y, Corradetti MN, Inoki K, Guan KL (2004) TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem Sci 29:32–38
Liu MY, Cai S, Espejo A, Bedford MT, Walker CL (2002) 14-3-3 interacts with the tumor suppressor tuberin at Akt phosphorylation site(s). Cancer Res 62:6475–6480
Louis M, Poortmans JR, Francaux M, Berre J, Boisseau N, Brassine E, Cuthbertson DJ, Smith K, Babraj JA, Waddell T, Rennie MJ (2003) No effect of creatine supplementation on human myofibrillar and sarcoplasmic protein synthesis after resistance exercise. Am J Physiol 285:E1089–E10894
MacKenna DA, Dolfi F, Vuori K, Ruoslahti E (1998) Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin-dependent and matrix-specific in rat cardiac fibroblasts. J Clin Invest 101:301–310
Markuns JF, Wojtaszewski JF, Goodyear LJ (1999) Insulin and exercise decrease glycogen synthase kinase-3 activity by different mechanisms in rat skeletal muscle. J Biol Chem 274:24896–24900
Mayer C, Zhao J, Yuan X, Grummt I (2004) mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev 18:423–434
Meijer AJ, Dubbelhuis PF (2004) Amino acid signalling and the integration of metabolism. Biochem Biophys Res Commun 313:397–403
Mordier S, Deval C, Bechet D, Tassa A, Ferrara M (2000) Leucine limitation induces autophagy and activation of lysosome-dependent proteolysis in C2C12 myotubes through a mammalian target of rapamycin-independent signaling pathway. J Biol Chem 275:29900–29906
Nader GA, Esser KA (2001) Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J Appl Physiol 90:1936–1942
Nave BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR (1999) Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J 344(2):427–431
Nellist M, Goedbloed MA, Halley DJ (2003) Regulation of tuberous sclerosis complex (TSC) function by 14-3-3 proteins. Biochem Soc Trans 31:587–591
Nielsen JN, Mustard KJ, Graham DA, Yu H, MacDonald CS, Pilegaard H, Goodyear LJ, Hardie DG, Richter EA, Wojtaszewski JF (2003) 5′-AMP-activated protein kinase activity and subunit expression in exercise-trained human skeletal muscle. J Appl Physiol 94:631–641
Noda T, Ohsumi Y (1998) Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem 273:3963–3966
Nojima H, Tokunaga C, Eguchi S, Oshiro N, Hidayat S, Yoshino K, Hara K, Tanaka N, Avruch J, Yonezawa K (2003) The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem 278:15461–15464
Pallafacchina G, Calabria E, Serrano AL, Kalhovde JM, Schiaffino S (2002) A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc Natl Acad Sci U S A 99:9213–9218
Parkington JD, Siebert AP, LeBrasseur NK, Fielding RA (2003) Differential activation of mTOR signaling by contractile activity in skeletal muscle. Am J Physiol 285:R1086–R1090
Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR (1998) Bidirectional modulation of insulin action by amino acids. J Clin Invest 101:1519–1529
Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR (1997) Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol 273:E99–E107
Phillips SM, Tipton KD, Ferrando AA, Wolfe RR (1999) Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol 276:E118–E124
Proud CG (2002) Regulation of mammalian translation factors by nutrients. Eur J Biochem 269:5338–5349
Qian Y, Corum L, Meng Q, Blenis J, Zheng JZ, Shi X, Flynn DC, Jiang BH (2004) PI3K induced actin filament remodeling through Akt and p70S6K1: implication of essential role in cell migration. Am J Physiol 286:C153–C163
Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR (2000) An oral essential amino acid–carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol 88:386–392
Raught B, Gingras AC, Sonenberg N (2001) The target of rapamycin (TOR) proteins. Proc Natl Acad Sci U S A 98:7037–7044
Rennie MJ, Wackerhage H (2003) Connecting the dots for mechanochemical transduction in muscle. J Physiol (Lond) 553:1
Sakamoto K, Hirshman MF, Aschenbach WG, Goodyear LJ (2002) Contraction regulation of Akt in rat skeletal muscle. J Biol Chem 277:11910–11917
Sakamoto K, Aschenbach WG, Hirshman MF, Goodyear LJ (2003) Akt signaling in skeletal muscle: regulation by exercise and passive stretch. Am J Physiol 285:E1081–E1088
Sakamoto K, Arnolds DE, Ekberg I, Thorell A, Goodyear LJ (2004) Exercise regulates Akt and glycogen synthase kinase-3 activities in human skeletal muscle. Biochem Biophys Res Commun 319:419–425
Sarbassov dos D, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM (2004) Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14:1296–1302
Serrano AL, Murgia M, Pallafacchina G, Calabria E, Coniglio P, Lomo T, Schiaffino S (2001) Calcineurin controls nerve activity-dependent specification of slow skeletal muscle fibers but not muscle growth. Proc Natl Acad Sci U S A 98:13108–13113
Shah OJ, Anthony JC, Kimball SR, Jefferson LS (2000) 4E-BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am J Physiol 279:E715–E729
Shah SA, Potter MW, Ricciardi R, Perugini RA, Callery MP (2001) FRAP-p70s6K signaling is required for pancreatic cancer cell proliferation. J Surg Res 97:123–130
Shaw M, Cohen P, Alessi DR (1998) The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem J 336(1):241–246
Sherwood DJ, Dufresne SD, Markuns JF, Cheatham B, Moller DE, Aronson D, Goodyear LJ (1999) Differential regulation of MAP kinase, p70(S6K), and Akt by contraction and insulin in rat skeletal muscle. Am J Physiol 276:E870–E878
Shumway SD, Li Y, Xiong Y (2003) 14-3-3beta binds to and negatively regulates the tuberous sclerosis complex 2 (TSC2) tumor suppressor gene product, tuberin. J Biol Chem 278:2089–2092
Siles-Lucas Mdel M, Gottstein B (2003) The 14-3-3 protein: a key molecule in parasites as in other organisms. Trends Parasitol 19:575–581
Stolovich M, Tang H, Hornstein E, Levy G, Cohen R, Bae SS, Birnbaum MJ, Meyuhas O (2002) Transduction of growth or mitogenic signals into translational activation of TOP mRNAs is fully reliant on the phosphatidylinositol 3-kinase-mediated pathway but requires neither S6K1 nor rpS6 phosphorylation. Mol Cell Biol 22:8101–8113
Sutherland C, Leighton IA, Cohen P (1993) Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J 296(1):15–19
Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J (2002) Tuberous sclerosis complex-1 and −2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci U S A 99:13571–13576
Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J (2003) Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol 13:1259–1268
Tipton KD, Wolfe RR (1998) Exercise-induced changes in protein metabolism. Acta Physiol Scand 162:377–387
Tipton KD, Rasmussen BB, Miller SL, Wolf SE, Owens-Stovall SK, Petrini BE, Wolfe RR (2001) Timing of amino acid–carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am J Physiol 281:E197–206
Wang L, Wang X, Proud CG (2000) Activation of mRNA translation in rat cardiac myocytes by insulin involves multiple rapamycin-sensitive steps. Am J Physiol 278:H1056–H1068
White MF, Maron R, Kahn CR (1985) Insulin rapidly stimulates tyrosine phosphorylation of a Mr-185,000 protein in intact cells. Nature 318:183–186
Wojtaszewski JF, Nielsen P, Kiens B, Richter EA, Wojtazsewski JF (2001) Regulation of glycogen synthase kinase-3 in human skeletal muscle: effects of food intake and bicycle exercise. Diabetes 50:265–269
Wolfe RR (2000) Protein supplements and exercise. Am J Clin Nutr 72:551S–557S
Wong TS, Booth FW (1990) Protein metabolism in rat tibialis anterior muscle after stimulated chronic eccentric exercise. J Appl Physiol 69:1718–1724
Xu G, Kwon G, Marshall CA, Lin TA, Lawrence JC Jr, McDaniel ML (1998) Branched-chain amino acids are essential in the regulation of PHAS-I and p70 S6 kinase by pancreatic beta-cells. A possible role in protein translation and mitogenic signaling. J Biol Chem 273:28178–28184
Yonezawa K (2003) Identification of TOR-interacting proteins. Mol Interv 3:189–193
Yoshizawa F, Nagasawa T, Nishizawa N, Funabiki R (1997) Protein synthesis and degradation change rapidly in response to food intake in muscle of food-deprived mice. J Nutr 127:1156–1159
Yoshizawa F, Kimball SR, Vary TC, Jefferson LS (1998) Effect of dietary protein on translation initiation in rat skeletal muscle and liver. Am J Physiol 275:E814–E820
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deldicque, L., Theisen, D. & Francaux, M. Regulation of mTOR by amino acids and resistance exercise in skeletal muscle. Eur J Appl Physiol 94, 1–10 (2005). https://doi.org/10.1007/s00421-004-1255-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-004-1255-6