Abstract
Heart rate variability (HRV) was assessed during the short- (within 1 h) and long- (within 48 h) term recovery following a single bout of either constant (CST) or interval training (SWEET) exercise performed at the same total physical work [9.4 (0.3) kJ kg−1]. R-R intervals, systolic (SAP) and diastolic (DAP) arterial pressures were recorded in supine and upright positions before and 1, 24 and 48 h after the termination of the exercises in ten male subjects [mean (SEM), age 24.6 (0.6) years, height 177.2 (1.1) cm and body mass 68.5 (0.9) kg]. The parameters were also recorded in the supine position during the first 20 min following the end of the exercise. Spectral analysis parameters of HRV [total (TP), low- (LF), and high- (HF) frequency power, and LF/TP, HF/TP and LF/HF ratios] were determined over 5 min during each phase. Except for higher HF values in both supine and upright positions during the first hour following CST compared with SWEET, cardiovascular and HRV analysis responses were of the same magnitude after their termination. R-R intervals, TP, and HF/TP were significantly decreased while LF/TP and LF/HF were significantly increased during the early recovery, when compared with control values. This could be a response to the significant decrease in SAP and DAP at this time. Twenty-four and 48 h after the end of the exercise, HRV parameters were at the same levels as before exercises in the supine posture, but a persistent tachycardia continued to be observed in the upright posture, together with reduced TP values, showing that cardiovascular functions were still disturbed. The short-term HRV recovery seemed dependent on the type of exercise, contrary to the long-term recovery.
Similar content being viewed by others
Introduction
As instantaneous heart rate depends on the interaction between sympathetic and parasympathetic activities and pacemaker properties, it has been suggested that the analysis of heart rate variability (HRV) could lead to a non-invasive assessment of the tonic autonomic regulation of heart frequency (Akselrod et al. 1981). Numerous studies have focused on the effects of exercise and exercise training on HRV. It seems established that exercise could be referred to as a ‘two-edged sword’ (Maron 2000). Indeed, regular physical activity improves indicators of autonomic function, including HRV and baroreflex sensitivity (Iellamo et al. 2000; Curtis and O’Keefe 2002; Mourot et al. 2004). However, the risk of sudden death is increased during and immediately after exercise (Albert et al. 2000), possibly related to acute changes in autonomic tone (Curtis and O’Keefe 2002). Exercise is associated with increased sympathetic tone and parasympathetic withdrawal, leading to a decreased HRV (Arai et al. 1989; Nakamura et al. 1993; Yamamoto et al. 1991), which has been related to an increased risk of cardiac disease and mortality (Curtis and O’Keefe 2002).
Data concerning the kinetic of recovery of autonomic function after the cessation of exercise are scarce. During the short-term recovery (within 30 min after the termination of exercise), the decrease of heart rate toward resting baseline seems mainly related to the prompt resumption of parasympathetic activity to the sinus node (Perini et al. 1989), and recovery of the autonomic regulation of heart rate has been proposed to occur within a few minutes after about 20 min of short-term intense exercise (Arai et al. 1989). A persistent sympathetic predominance has also been observed 1 h after the cessation of the exercise and pre-exercise levels appeared to be regained only 24 h after the exercise bout (Furlan et al. 1993; Bernardi et al. 1997). A rebound of the parasympathetic activity was also found 2 days after prolonged exercise (Hautala et al. 2001). However, because of their length and the environmental conditions, the exercises performed in the aforementioned studies differ from those that individuals would routinely perform in an endurance training programme. Indeed, changes in HRV were reported after 30 min of maximal exercise leading to exhaustion (Furlan et al. 1993), 46 km of running at a mean altitude of 2,500 m (Bernardi et al. 1997), and 75 km of cross-country skiing (Hautala et al. 2001).
Interval training involves repeated short to long bouts of rather high intensity exercise interspersed with recovery periods (light exercise or rest; Billat 2001). It has been reported that interval training, compared with constant aerobic training sessions, results in relatively greater improvements in the exercise intensity and respiratory parameters not only in healthy subjects, but also in heart transplant recipients (Maslowsky et al. 1994). Despite the reported advantages of interval training in increasing aerobic capacity and performances (Fox et al. 1975) and its growing utilisation in the training sessions (Gimenez et al. 1982; Geny et al. 1996; Tordi et al. 2001a,b), the majority of exercise and/or of endurance training programmes have studied HRV using constant exercise (e.g. Arai et al. 1989; Melanson and Freedson 2001).
Therefore, the aims of the present study were: (1) to assess the short- (within 1 h) and long- (within 48 h) term HRV recovery after a single bout of exercise, and (2) to assess whether the recovery of HRV is altered by the type of exercise performed (i.e. interval training or constant exercise). The total physical work performed during each session was adjusted to remain equal whatever the type of exercise. These exercises were chosen in order to correspond to those that many subjects might routinely perform during a training session, even when rehabilitation procedures are considered.
Methods
Subjects
Ten healthy, moderately trained men participated in the study [mean (SEM), age 24.6 (0.6) years, height 177.2 (1.1) cm and body mass 68.5 (0.9) kg]. Medical histories and medical examinations were used to reject subjects with cardiovascular, pulmonary, or metabolic diseases. The subjects were normotensive and were not taking any medication. All the subjects participated in the study voluntarily and provided written informed consent, which was approved by the local ethics committee. Subjects were instructed to fast for at least 3 h before testing, to refrain from ingesting beverages containing caffeine and alcohol for at least 24 h prior to testing, and not to exercise (beyond normal lifestyle activities) during the 48 h preceding the test sessions and between the exercise and the end of the recovery study procedure (48 h after the end of the exercise).
Study protocol
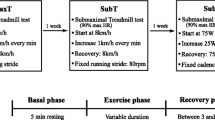
The subjects performed three exercises at a constant pedalling rate of 80 rpm on a mechanically braked cycle ergometer (Monark 818E, Stockholm, Sweden), separated by at least 7 days, and at the same time of the day. All the subjects started the study by performing an incremental test in order to determine the power output achieved at peak and ventilatory threshold (VT) levels. Thereafter, they performed the interval training [square-wave endurance exercise test (SWEET)] and the constant exercises in a random order.
Cardiovascular signals (heart rate, systolic and diastolic blood pressures) were measured with the same technique over a 10-min period while the subject was in the supine position and over 10 min in the upright position before, and 1 h (E+1 h), 24 h (E+24 h), and 48 h (E+48 h) after the cessation of the exercise. The immediate response at the end of the exercise was also assessed over a 20-min period, with the subjects in the supine position. In this case, analyses during the first 5 min after the cessation of the exercise were discarded, and analyses were performed every 5 min, i.e. (1) from 5 to 10 min, (2) from 10 to 15 min, and (3) from 15 to 20 min after the end of exercise. The room was quiet, with dim lighting and the ambient temperature was kept between 21°C and 24°C.
Incremental exercise
The subjects were connected to a gas analyser system (Medical graphics type CPX/D, St. Paul, Minn.), which was calibrated using gases of known concentration. Ventilatory variables were averaged every 30 s. The respiratory and metabolic variables were oxygen consumption (V̇O2, millilitres per kilogram per minute), carbon dioxide production (V̇CO2, millilitres per kilogram per minute), respiratory exchange ratio [R, (V̇CO2/V̇O2)], minute ventilation (V̇ E, litres per minute), tidal volume (V T, litres), and breathing frequency (f, breaths per minute). Incremental exercise was performed after a resting period of 3 min with the subjects seated on the ergometer. After cycling at 150 W for 3 min, a 30-W load was added every 3 min until exhaustion (Gimenez et al. 1982). The highest load that could be maintained at a pedalling rate of 80 rpm for 3 min was taken as the maximal power output (Ẇ M, Watts). Peak oxygen uptake (V̇O2p) and peak R-R intervals (RRp, milliseconds) were determined at Ẇ M (averaged over the last 30 s). VT was assessed from the relationships between R, V̇ E, V̇ E/V̇O2 ratio, V̇ E/V̇CO2 ratio, and power output (Wasserman 1986) by three experts blind with respect to the condition they were evaluating. The mean of the two measurements that were closest in value was taken as the VT, and the corresponding oxygen uptake (V̇O2VT), R-R intervals (RRVT), and power output (Ẇ VT) were registered (averaged values over 30 s).
SWEET exercise
SWEET was performed according to the recommendations given by Gimenez et al. (1982). SWEET consists of periods of maximal intensity superimposed to a submaximal ‘base’ exercise. Specifically, a session comprises nine consecutive periods of 5 min including 4 min ‘base’ work-rate followed by 1 min ‘peak’ work-rate initially performed at the Ẇ VT and Ẇ M levels, respectively.
Constant exercise
The constant exercise (CST) was performed at Ẇ VT. The duration was adjusted in order to allow the subject to perform the same total physical work (TPW, kilojoules per kilogram) as during SWEET.
During SWEET and CST exercises, the mean R-R interval was calculated as the averaged value of the R-R intervals of the entire exercise. The mean R-R interval at the end of each exercise was averaged over the last 30 s.
Measurements
During all recordings, the subjects spontaneously adapted their VT and f; the latter was recorded thanks to a respiratory amplifier consisting of a chest belt that measures the changes in thoracic circumference while breathing (Biopac System, Santa Barbara, Calif.). In the present study, f was always >10 cycles min−1 (0.16 Hz) and no significant differences were observed between the different conditions. Despite previously reported controversial results, no effects of f and VT on HRV assessment were found in the present study (Brown et al. 1993; Cooke et al. 1998).
Signal processing
The ECG was sampled at 500 Hz using an analogue to digital converter with 16-bit resolution and data acquisition software (BSL pro v.3.6.5., Biopac System) that calculated the R-R intervals as the difference between successive peaks. All the R-R intervals were edited initially by visual inspection to exclude all the undesirable beats (i.e. to ensure that each analysis for the segment was free of movement artefact and/or sharp transient in the signal due to premature beats), which counted for <1% in every subject.
Frequency domain analyses were performed on a time series of five consecutive 1-min periods manually selected over the 10-min recordings in the supine and standing postures (before, E+1 h, E+24 h, and E+48 h, and during the 20 min after the cessation of the exercise). At least 256 cycles were used for each analysis. Spectral analysis was performed with the coarse graining spectral analysis (CGSA) method which separates the harmonic components of interest from the non-harmonic, or fractal, noise components that occur primarily in the very-low-frequency region, achieving better spectral estimates especially with short-term ECG recordings (Yamamoto and Hughson 1991, 1994). The total harmonic power of HRV (TP) and the power of spectral components in the low (LF) and high frequencies (HF) were quantified as follows: general spectral analysis (GSA) utilises the total spectra from 0 to 0.5 Hz with subdivisions of LF power from 0 to 0.15 Hz and 0.15 to 0.5 Hz for HF power (Yamamoto et al. 1991). GSA divides the data into three equal parts for the mathematical requirement of equal distance intervals for fast Fourier transforms (FFT). FFT is applied with the ensemble averages subjected to a Hanning-type spectral window to produce HRV spectra (Yamamoto and Hughson 1991). This mathematical procedure delineates the HRV signal from the time to frequency domain, where the LF and HF power can be distinguished. CGSA is a modified version of GCA that subtracts the non-harmonic component from the original spectrum (Yamamoto and Hughson 1991). Elimination of the non-harmonic (fractal) components leaves the harmonic component intact for identification of power spectra content, which is in a manner consistent with previous recommendations (Yamamoto and Hughson 1991; Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology 1996). HF power is almost entirely mediated by the parasympathetic activity to the sinus node directly associated with respiratory activity (Pomeranz et al. 1985; Yamamoto et al. 1995), whereas LF power reflects the mixed modulation of parasympathetic and sympathetic activities (Bernardi et al. 1994). Modulation of sympathetic and parasympathetic nervous system activities appears to be best characterised by normalising the relative distribution of spectral power in the LF and HF regions (Pagani and Malliani 2000). Parasympathetic nervous system activity was thus evaluated by HF and HF/TP ratio whereas sympathetic nervous system activity was evaluated by LF/TP and LF/HF ratios (Yamamoto and Hughson 1991; Yamamoto et al. 1991, 1995; Nakamura et al. 1993). Although computation of the absolute power in these regions differs with technique (i.e. CGSA compared with FFT and autoregressive spectral analysis), the ratio of LF/HF generally reflects changes in the contribution of sympathetic nervous system to HRV even if LF/HF has been also labelled as an index of sympathovagal balance (Pagani et al. 1986).
SAP and DAP were measured every 3 min at the brachial level using an automated device (BP-8800, Colin Electronics, Japan). The mean of the two closest values is reported. Mean arterial pressure (MAP) was calculated as MAP=DAP+1/3(SAP−DAP).
The difference in autonomic markers (R-R intervals, TP, LF, HF, LF/TP, HF/TP, and LF/HF) and blood pressures (SAP, DAP) in the two positions before and at the different times of recovery (E+1 h, E+24 h, and E+48 h) were calculated (as percentages).
Statistical analysis
Data are expressed as means (SEM). Statistical analysis was performed by means of the Sigma Stat package (2.03, SPSS, San Rafael, Calif.). The Gaussian distribution of the data was verified by the Kolmogorov–Smirnov goodness-of-fit test (Z value <1.0). Because the HRV spectral analysis indexes were skewed, they were transformed into their logarithms (ln). Differences between recovery times after SWEET and CST were evaluated using a one-way repeated measures ANOVA. Differences between SWEET and CST for each measurement were evaluated with a paired t-test. Significant F ratio differences were identified by the Newman–Keuls test. The statistical significance was established at the P<0.05 level.
Results
Exercises
At the end of the incremental exercises, V̇O2p, RRp, and Ẇ M were 61.3 (1.1) ml kg−1min−1, 327 (5) ms, and 308 (6) W, respectively. At the VT level, V̇O2VT, RRVT, and Ẇ VT were 48.3 (1.1) ml kg−1min−1, 369 (8) ms, and 228 (5) W, respectively.
During SWEET, the mean power performed was 228 (5) W and 308 (6) W at base and peak levels, respectively, corresponding to a mean TPW of 9.4 (0.3) kJ kg−1. The duration of CST was thus 48.2 min [i.e. 45+3.2 (0.3) min], performed at a mean power output of 228 (5) W. The mean R-R interval was lower during SWEET than during CST [360 (87) ms and 374 (45) ms, respectively; P<0.05], and the R-R interval at the end of SWEET was significantly lower than at the end of CST [341 (46) ms and 360 (46) ms, respectively; P<0.05].
Before the exercises
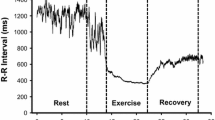
When SWEET was compared with CST, no significant differences were found in R-R intervals, blood pressures, or cardiac autonomic control values, whatever the posture (Tables 1 and 2; Fig. 1). Compared with the supine posture, the upright position induced an increase in LF, LF/TP, and LF/HF, and a decrease in R-R interval, TP, HF, and HF/TP (Table 1; Fig. 1). DAP and MAP increased significantly with the upright posture (Table 2).
Sympathetic and parasympathetic nervous activity evaluated by spectral analysis of heart rate variability (HRV) before and in short- (within 1 h) and long- (within 48 h) term recovery following interval training (SWEET) and constant (CST) exercises. HRV was evaluated in supine and upright postures at each time except during three 5-min periods of the 20 min following the termination of the exercise, where the subjects were evaluated in the supine posture only. TP Total power of heart rate variability, LF low-frequency power of heart rate variability, HF high-frequency power of heart rate variability. §Significantly different value from that for the corresponding supine posture at the P<0.05 level, *significantly different value from that for the supine posture before exercise at the P<0.05 level
Short-term exercise effects
During the 20 min following the cessation of the exercise, an important decrease in R-R interval, TP, LF, HF, and HF/TP values and an increase in LF/TP and LF/HF were observed, after both SWEET and CST (P<0.05; Table 1; Fig. 1). Blood pressures values decreased after the two exercises but a significant level was reached only for SAP (Table 2). No significant differences were observed between the three ‘5-min periods’ of the short-term recovery, except a progressive increase in HF (P<0.05; Table 1) and LF values (P<0.05 after SWEET only; Table 1). After CST compared with after SWEET, higher TP values (P<0.05; 5 min after the cessation of exercise) and higher HF values (P<0.05; during the entire 20-min period) were observed (Table 1).
At E+1 h, in the supine position, R-R interval, PAS, PAD, PAM, TP, and LF values were lower than before exercise (P<0.05), whatever the exercise. HF values were significantly lower and LF/TP was significantly higher after SWEET but remained unchanged after CST (Tables 1, 2; Fig. 1). TP and HF values were higher after CST than after SWEET at the same time in this posture (Table 1).
Compared with the supine posture, the upright position induced a significant decrease in R-R, HF, and HF/TP. TP decreased 1 h after exercise but a significant level was reached after CST only. PAS, PAD, PAM, LF, LF/TP, and LF/HF (Tables 1, 2; Fig. 1), increased significantly in the upright posture, after both SWEET and CST. At E+1 h in the upright posture, HF remained lower after SWEET than after CST.
R-R interval, PAS, PAD, PAM, TP, LF, and HF values were lower in the upright position at E+1 h than in the upright position before exercise (P<0.05; Tables 1, 2; Fig. 1).
Long-term exercise effects
At E+24 h and E+48 h, (Tables 1, 2; Fig. 1), supine values of blood pressures and autonomic cardiovascular control parameters were equal to those before exercise. The upright position induced a decrease in R-R interval, TP, HF, and HF/TP (P<0.05) and an increase in LF/TP and LF/HF (P<0.05 only at E+24 h after CST). At E+24 h, the values of SAP, DAP, and MAP remained unchanged in the upright compared with the supine posture, whereas they increased at E+48 h (Table 2; P<0.05). Significant lower R-R interval values were observed in the upright posture after SWEET at E+24 h, and after the two exercises at E+48 h, compared with control values. No differences in cardiac autonomic control and blood pressures values were noted between the two kinds of exercises.
Upright posture-induced relative changes
No significant differences were observed between the posture-induced relative changes, whatever the time of recovery or type of exercise (Table 3).
Discussion
Despite the important reported link between the autonomic nervous system (which can be evaluated with HRV analysis) and possible cardiovascular events during exercise and after its termination, only few studies have examined the long-term kinetics of HRV recovery after exercise. Moreover, the majority of studies have focused on HRV and exercise or training using constant exercise. In the present study, we examined the short- (within 1 h) and long- (within 48 h) term kinetics of HRV recovery after both SWEET and CST exercise performed at the same level of total physical work.
Early recovery (from 5 min to 1 h after the termination of the exercise)
The size and pattern of recovery in the early period following the cessation of the exercise were approximately the same after SWEET and CST, and are consistent with published studies. Perini et al. (1990) suggested that during the first 10 min after the termination of a 5-min exercise at low intensity (below 30% of maximal oxygen consumption), and after the initial fast heart rate transient elapsed, a power distribution similar to the pre-exercise period was already observed suggesting that mechanisms controlling the cardiovascular system and modified by the exercise had returned to resting conditions within 5 min. When intensity increased (higher than 30% of maximal oxygen consumption), HF remained lower, and LF and LF/HF were higher compared with baseline levels (Hayashi et al. 1992; Terziotti et al. 2001). This trend reflected the slow return of sympathetic activity to resting values (Perini et al. 1989, 1990), and thus resting conditions were not achieved within 5 min (Perini et al. 1990), 9 min (Arai et al. 1989), or even 1 h (Hayashi et al. 1992; Terziotti et al. 2001) after the termination of the exercise, depending on its intensity and duration. In the present study, the decrease in R-R interval values (Furlan et al. 1993; Bernardi et al. 1997; Terziotti et al. 2001; James et al. 2002) and in the frequency domain analysis indexes of HRV (decrease in TP, HF, LF, and HF/TP values, increase in LF/TP and LF/HF ratios) at E+1 h after both SWEET and CST exercises were consistent with these previous findings and with the same decrease in HRV observed after a single bout of interval training (James et al. 2002).
During the first hour following exercise, there was a significantly more important decrease in TP values between 5 and 10 min of recovery and in HF values between 5 min and E+1 h of recovery after SWEET, compared with CST, showing a slower return of parasympathetic activity during the short-term recovery after SWEET. This slower return could be due to a more pronounced withdrawal of parasympathetic activity and/or more important sympathetic involvement during SWEET compared with CST. The power outputs at ‘base’ and ‘peak’ levels were chosen in order to trigger a drift in R-R intervals and to achieve almost 90% of HRp at the end of exercise (Gimenez et al. 1982). Thus, for the same total physical work performed, the mean R-R interval values and the R-R interval values at the end of SWEET were lower when compared with CST. An increase in heart rate, or a decrease in R-R interval values, reflect parasympathetic withdrawal and sympathetic predominance (Goldberger 1999), and, despite the fact that we did not evaluate the autonomic nervous system activity (e.g. plasma catecholamine concentrations) and local anaerobic metabolites during the exercise, we thought that parasympathetic activity was more depressed and/or sympathetic activity was more involved during SWEET than during CST. Thus, the short-term recovery seemed to depend on the type of exercise (i.e. interval training vs constant) and not on the total physical work performed.
The decrease in R-R interval values and in HRV in supine and upright postures during the first hour of recovery could be a response to the significantly lower blood pressures, compared with control values (significant decrease in SAP values and trend for a decrease in MAP values during the first 20 min, and significant decrease of SAP, DAP, and MAP at E+1 h; Kenney and Seals 1993; Piepoli et al. 1993; Raine et al. 2001). The reduced blood pressures could not be explained by the data collected in the present study, but several hypotheses have been reported in the literature. A decrease in sympathetic drive, as demonstrated by reduced norepinephrine responses, reduced the blood pressure responses to stress after acute exercise (Brownley et al. 2003). In the present study, sympathetic indexes (LF/TP, LF/HF) were not significantly different at E+1 h compared with control values, suggesting that the sympathetic activity was unchanged. Moreover, the upright posture increased LF/TP and LF/HF values, and decreased HF and HF/TP values, which indicated that the sympathetic nervous system could still be increased, as before exercise and at E+24 h and E+48 h (Table 3). Bernadi et al. (1997) observed that the effect of sympathetic modulation of vessels was partially preserved 30 min after 46 km of running at 2,500 m of altitude exercise; this was nevertheless insufficient to counteract the post-exercise hypotension. In the study of Raine et al. (2001), the capacity for peripheral vasoconstriction was not utilised to return blood pressure back to baseline values in the supine position, whereas this was the case in seated posture. We found lower values of blood pressures in both supine and upright postures, but the capacity for vasoconstriction seemed to be still operational since the subjects increased their blood pressures during the orthostatism challenge (Table 2). It is possible that the arterial baroreceptor reflex was reset to a lower operating pressure after exercise, together with an increase in the baroreflex responsiveness (Halliwill et al. 1996; Raine et al. 2001).
Late recovery (24 h and 48 h after the termination of the exercise)
Long-term effects (within 24 h to 72 h) of a single bout of exercise have rarely been studied. Despite the mean R-R interval returning to control value, Furlan and al. (1993) have observed a predominance of the LF components 24 h after the termination of the exercise in supine position, the baseline values being reached only after 48 h. Since in their study, endurance-trained athletes had higher LF values than control subjects, the authors hypothesised that the training routine per se could lead to an after-effect of the previous day’s activity, leading to an increase in sympathetic modulation to the sinus node, while leaving heart rate unaffected. However, their experiment was designed to study both the alteration of HRV by endurance training and by exercise, and the fact that LF values were higher in athletes than in control subjects has been paralleled with the possibility of an overtraining status (Piepoli and Coats 1994), even though the authors explained that it could not have been possible (Furlan et al. 1994).
Differently, the biphasic autonomic effects of a 43-km run at altitude showed that immediately after completion of the exercise, moderate sympathetic predominance and baroreceptor efferent activity via the sympathetic branch were evident; 24 h later there was a reversal in baroreceptor efferent activity with enhancement of the parasympathetic branch, whereas the sympathetic component of the response was attenuated (Bernardi et al. 1997). In another study, this rebound of parasympathetic activity was observed only 48 h after 75 km of cross-country skiing (Hautala et al. 2001). In the present study, we did not observe such a phenomenon. The training status of the subjects could explain these discrepancies. The subjects of the previous studies performed several hours of intense exercise in extreme conditions and were very well trained, at least more highly trained than the subjects of the present study. In the studies of Bernardi et al. (1997) and Hautala et al. (2001), the exercises were not comparable with the exercises performed in the present study because of their length and degree of difficulty. The only published data available for a single bout of interval training reported HRV values 1 h and 72 h after the termination of the exercise and thus the time course of the changes during the 72 h was unknown (James et al. 2002).
The results of the present study indicated that the long-term HRV recovery was the same after SWEET and after CST. Therfore, it appears that it is the total physical work performed during the exercise that determines the long-term HRV recovery, and not the type of exercise (i.e. interval training or constant exercise), contrary to the short-term HRV recovery. Moreover, 24 h after SWEET or CST, the changes in cardiovascular autonomic control were reversed to baseline in the supine position. On the contrary, in the upright posture, we observed a significant drop in mean R-R interval at E+24 h and E+48 h. These differences were accompanied by a decrease in TP (not significant at E+24 h after CST; Table 1). Based on the results of Goldberger et al. (1999), we are able to hypothesise a sympathetic predominance during the upright posture; however, no significant changes were observed in LF, HF, LF/TP, HF/TP, and LF/HF, compared with control values. At E+24 h, blood pressures were not increased with the upright posture, whereas they were before the exercise and at E+1 h and E+48 h (Table 2). It is not possible to explain these two phenomena from the present HRV analysis. Bernardi et al. (1997) reported changes in R-R interval and HRV values after exercise in both supine and upright positions 24 h and 48 h after the end of an exhaustive race. They observed a decrease in blood pressure as long as 24 h after the end of the exercise, but R-R interval values were at the same level as before the race. They performed neck suction stimulation at a low frequency (0.10 Hz) which became ineffective in modulating SAP and DAP at 24 h, indicating reduced sensitivity of the arterial vessels to sympathetic stimulation, but this stimulation remained effective on R-R interval values similar to baseline. Data concerning long-term HRV recovery after exercise are sparse and, to our knowledge, it is the first time that such a postural tachycardia has been observed as long as 48 h after the cessation of an exercise. Further research may be needed to confirm these results, to explain the tachycardia, and to find out whether the depressed HRV after exercise has any importance in the occurrence of cardiac events.
In the present study, healthy subjects were examined in both supine and upright postures after a single bout of either SWEET or CST. Results showed that autonomic cardiovascular control is shifted to sympathetic predominance for at least 1 h after cessation of the exercise, but the responses to orthostatism were maintained. The depressed HRV and enhanced sympathetic activity observed 1 h after the exercise should be a reflex response to the persistent drop in blood pressures, which may be due to a resetting of the arterial baroreceptor reflex. Despite the same total physical work performed, SWEET led to a more depressed parasympathetic activity to the sinus node during the first hour after the end of the exercise. The more important involvement of sympathetic activity during SWEET (due to the alternation of low- and high-intensity bouts of exercise) could explain this lag. The recovery of HRV values was achieved in 24 h in the supine position, but R-R intervals and TP were decreased in the upright posture as long as 48 h after the cessation of the exercise. No parameters of spectral analysis of HRV suggested a change in sympathovagal balance at this time.
References
Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ (1981) Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 213:220–222
Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE (2000) Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med 343:1355–1361
Arai Y, Saul JP, Albrecht P, Hartley LH, Lilly LS, Cohen RJ, Colucci WS (1989) Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol 256:H132–H141
Bernardi L, Leuzzi S, Radaelli A, Passino C, Johnston JA, Sleight P (1994) Low-frequency spontaneous fluctuations of R-R interval and blood pressure in conscious humans: a baroreceptor or central phenomenon? Clin Sci (Lond) 87:649–654
Bernardi L, Passino C, Robergs R, Appenzeller O (1997) Acute and persistent effects of a 46-kilometer wilderness trail run at altitude: cardiovascular autonomic modulation and baroreflexes. Cardiovasc Res 34:273–280
Billat LV (2001) Interval training for performance: a scientific and empirical practice. Special recommendations for middle- and long-distance running. Part I: aerobic interval training. Sports Med 31:13–31
Brown TE, Beightol LA, Koh J, Eckberg DL (1993) Important influence of respiration on human R-R interval power spectra is largely ignored. J Appl Physiol 75:2310–2317
Brownley KA, Hinderliter AL, West SG, Girdler SS, Sherwood A, Light KC (2003) Sympathoadrenergic mechanisms in reduced hemodynamic stress responses after exercise. Med Sci Sports Exerc 35:978–986
Cooke WH, Cox JF, Diedrich AM, Taylor A, Beightol LA, Ames IV JE, Hoag JB, Seidel H, Eckberg DL (1998) Controlled breathing protocols probe human autonomic cardiovascular rythms. Am J Physiol 274:H709–H718
Curtis BM, O’Keefe JH Jr (2002) Autonomic tone as a cardiovascular risk factor: the dangers of chronic fight or flight. Mayo Clin Proc 77:45–54
Fox EL, Bartels RL, Billings CE, O’Brien R, Bason R, Mathews DK (1975) Frequency and duration of interval training programs and changes in aerobic power. J Appl Physiol 38:481–484
Furlan R, Piazza S, Dell’Orto S, Gentile E, Cerutti S, Pagani M, Malliani A (1993) Early and late effects of exercise and athletic training on neural mechanisms controlling heart rate. Cardiovasc Res 27:482–488
Furlan R, Pagani M, Malliani A (1994) Effects of exercise on the autonomic control of the heart: training or overtraining? Cardiovasc Res 28:142–143
Geny B, Saini J, Mettauer B, Lampert E, Piquard F, Follenius M, Epailly E, Schnedecker B, Eisenmann B, Haberey P, Lonsdorfer J (1996) Effect of short-term endurance training on exercise capacity, haemodynamics and atrial natriuretic peptide secretion in heart transplant recipients. Eur J Appl Physiol 73:259–266
Gimenez M, Servera E, Salinas W (1982) Square-wave endurance exercise test (SWEET) for training and assessment in trained and untrained subjects. I. Description and cardiorespiratory responses. Eur J Appl Physiol 49:359–368
Goldberger JJ (1999) Sympathovagal balance: how should we measure it? Am J Physiol 276:H1273–H1280
Halliwill JR, Taylor JA, Hartwig TD, Eckberg DL (1996) Augmented baroreflex heart rate gain after moderate-intensity, dynamic exercise. Am J Physiol 270:R420–R426
Hautala A, Tulppo MP, Makikallio TH, Laukkanen R, Nissila S, Huikuri HV (2001) Changes in cardiac autonomic regulation after prolonged maximal exercise. Clin Physiol 21:238–245
Hayashi N, Nakamura Y, Muraoka I (1992) Cardiac autonomic regulation after moderate and exhaustive exercises. Ann Physiol Anthropol 11:333–338
Iellamo F, Legramante JM, Massaro M, Raimondi G, Galante A (2000) Effects of a residential exercise training on baroreflex sensitivity and heart rate variability in patients with coronary artery disease: A randomized, controlled study. Circulation 102:2588–2592
James DV, Barnes AJ, Lopes P, Wood DM (2002) Heart rate variability: response following a single bout of interval training. Int J Sports Med 23:247–251
Kenney MJ, Seals DR (1993) Postexercise hypotension. Key features, mechanisms, and clinical significance. Hypertension 22:653–664
Maron BJ (2000) The paradox of exercise. N Engl J Med 343:1409–1411
Maslowsky F, Anza C, Pedretti R, Santoro F, Bonelli R, Pribevich M, Caru B (1994) Effects of two types of physical training (aerobic versus anaerobic) in early cardiac trasnplantation (abstract). Eur Heart J 15:320
Melanson EL, Freedson PS (2001) The effect of endurance training on resting heart rate variability in sedentary adult males. Eur J Appl Physiol 85:442–449
Mourot L, Bouhaddi M, Perrey S, Rouillon JD, Regnard J (2004) Quantitative Poincare plot analysis of heart rate variability: effect of endurance training. Eur J Appl Physiol 91:79–87
Nakamura Y, Yamamoto Y, Muraoka I (1993) Autonomic control of heart rate during physical exercise and fractal dimension of heart rate variability. J Appl Physiol 74:875–881
Pagani M, Malliani A (2000) Interpreting oscillations of muscle sympathetic nerve activity and heart rate variability. J Hypertens 18:1709–1719
Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E (1986) Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 59:178–193
Perini R, Orizio C, Comande A, Castellano M, Beschi M, Veicsteinas A (1989) Plasma norepinephrine and heart rate dynamics during recovery from submaximal exercise in man. Eur J Appl Physiol 58:879–883
Perini R, Orizio C, Baselli G, Cerutti S, Veicsteinas A (1990) The influence of exercise intensity on the power spectrum of heart rate variability. Eur J Appl Physiol 61:143–148
Piepoli M, Coats AJ (1994) Effects of exercise on the autonomic control of the heart: training or overtraining? Cardiovasc Res 28:141–142
Piepoli M, Coats AJ, Adamopoulos S, Bernardi L, Feng YH, Conway J, Sleight P (1993) Persistent peripheral vasodilation and sympathetic activity in hypotension after maximal exercise. J Appl Physiol 75:1807–1814
Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Cohen RJ (1985) Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol 248:H151–H153
Raine NM, Cable NT, George KP, Campbell IG (2001) The influence of recovery posture on post-exercise hypotension in normotensive men. Med Sci Sports Exerc 33:404–412
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996). Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93:1043–1065
Terziotti P, Schena F, Gulli G, Cevese A (2001) Post-exercise recovery of autonomic cardiovascular control: a study by spectrum and cross-spectrum analysis in humans. Eur J Appl Physiol 84:187–194
Tordi N, Belli A, Mougin F, Rouillon JD, Gimenez M (2001a) Specific and transfer effects induced by arm or leg training. Int J Sports Med 22:517–524
Tordi N, Dugue B, Klupzinski D, Rasseneur L, Rouillon JD, Lonsdorfer J (2001b) Interval training program on a wheelchair ergometer for paraplegic subjects. Spinal Cord 39:532–537
Wasserman K (1986) The anaerobic threshold: definition, physiological significance and identification. Adv Cardiol 35:1–23
Yamamoto Y, Hughson RL (1991) Coarse-graining spectral analysis: new method for studying heart rate variability. J Appl Physiol 71:1143–1150
Yamamoto Y, Hughson RL (1994) On the fractal nature of heart rate variability in humans: effects of data length and beta-adrenergic blockade. Am J Physiol 266:R40–R49
Yamamoto Y, Hughson RL, Peterson JC (1991) Autonomic control of heart rate during exercise studied by heart rate variability spectral analysis. J Appl Physiol 71:1136–1142
Yamamoto Y, Nakamura Y, Sato H, Yamamoto M, Kato K, Hughson RL (1995) On the fractal nature of heart rate variability in humans: effects of vagal blockade. Am J Physiol 269:R830–R837
Acknowledgements
We especially thank the subjects who volunteered for this study. This work was funded by the granting of the EA 479 by the Minsitère de l’Education Nationale, de la Recherche et de la Technologie, France.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mourot, L., Bouhaddi, M., Tordi, N. et al. Short- and long-term effects of a single bout of exercise on heart rate variability: comparison between constant and interval training exercises. Eur J Appl Physiol 92, 508–517 (2004). https://doi.org/10.1007/s00421-004-1119-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-004-1119-0