Abstract
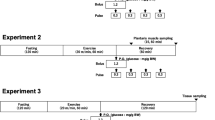
The purpose of the present study was to assess the effects of exogenously increasing the circulating levels of glucagon on the metabolic responses to exercise in rats. A total of six groups of rats were infused (iv) either with glucagon (20 or 50 ng·kg−1·min−1) or saline (0.9% NaCl), either in the resting state or during a bout of running exercise (45 min, 26 m·min−1, 0% grade). Blood samples were taken at the end of the 45-min experiment. Animals infused with glucagon at 50 ng·kg−1·min−1 showed significantly (P<0.01) higher mean plasma glucagon concentrations than animals infused with saline or glucagon at 20 ng·kg−1·min−1. In addition, exercise resulted in significantly (P<0.05) higher mean plasma glucagon concentrations, compared to rest, in all groups. In spite of these differences in glucagon concentrations, there were no significant (P>0.05) effects of exercise and glucagon infusion on mean hepatic glycogen, plasma glucose, insulin, C-peptide, β-hydroxybutyrate, or catecholamine concentrations. Although exercise resulted in a significant (P<0.01) increase in plasma glycerol and free fatty acid concentrations and a significant (P<0.05) decrease in glycogen in the soleus muscle, these responses were not affected by the glucagon infusion. These results suggest that the liver is non-responsive to physiological hyperglucagonemia in a short-term (45 min) exercise situation.




Similar content being viewed by others
References
Cherrington AD, Williams PF, Shulman GI, Lacy WW (1981) Differential time course of glucagon's effect on glycogenolysis and gluconeogenesis in the conscious dog. Diabetes 30:180–187
Delahousse M, Mercier O, Bichara M, Paillard M, Leviel F, Assan R (1988) Glucagon inhibits urinary acidification in the rat. Am J Physiol 254:F762–F769
Drouin R, Lavoie C, Bourque J, Ducros F, Poisson D, Chiasson JL (1998) Increased hepatic glucose production response to glucagon in trained subjects. Am J Physiol 274: E23–E28
Geary N, Le Sauter J, Noh U (1993) Glucagon acts in the liver to control spontaneous meal size in rats. Am J Physiol 264:R116–R122
Issekutz B, Vranic M (1980) Significance of glucagons in the control of glucose production during exercise. Am J Pysiol 238:E13–E20
Kabadi UM (1993) Hepatic regulation of pancreatic alpha-cell function. Metabolism 42:535–543
Lavoie J-M, Lord M, Paulin A (1988) Effect of selective hepatic vagotomy in plasma FFA levels in resting and exercising rats. Am J Physiol 254:R602–R606
Lo S, Russell JC, Taylor AW (1970) Determination of glycogen in small tissue samples. J Appl Physiol 28:234–236
Magnusson I, Tothman D, Gerard DP, Katz LD, Shulman GI (1995) Contribution of hepatic glycogenolysis to glucose production in humans in response to a physiological increase in plasma glucagon concentration. Diabetes 44:185–189
Matas Bonjorn V, Latour MG, Belanger P, Lavoie J-M (2002) Effects of exercise and liver glycogen content on hyperglucagonemia-induced hepatic glucose production in rats. J Appl Physiol 92:188–194
Rémie R, Zaagsma J (1986) A new technique for the study of vascular presynaptic receptors in freely moving rats. Am J Physiol 251:H463–H467
Tadjore M, Bergeron R, Latour M, Desy F, Warren C, Lavoie JM (1997) Effects of dietary manipulations and glucose infusion on glucagon response during exercise in rats. J Appl Physiol 83:148–152
Wasserman DH (1995) Regulation of glucose fluxes during exercise in the postabsorptive state. Annu Rev Physiol 57:191–218
Wasserman DH, Lickley HLA, Vranic M (1984) Interactions between glucagons and other counterregulatory hormones during normoglycemic and hypoglycemic exercise in dogs. J Clin Invest 74:1404–1413
Wasserman DH, Spalding JA, Lacy DB, Colburn CA, Goldstein RE, Cherrington AD (1989) Glucagon is a primary controller of hepatic glycogenolysis and gluconeogenesis during muscular work. Am J Physiol 257:E108–E117
Acknowledgements
This work was supported by the Natural Sciences and Engineering Research council of Canada and Fonds pour la Formation des Chercheurs et l'Aide à la Recherche (Government of Québec).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bélanger, P., Fillion, Y., Couturier, K. et al. Effects of inducing physiological hyperglucagonemia on metabolic responses to exercise. Eur J Appl Physiol 89, 8–13 (2003). https://doi.org/10.1007/s00421-002-0766-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-002-0766-2