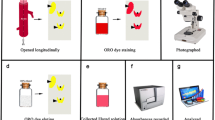
Abstract
En-face fat staining is frequently used to visualize atherosclerotic lesions. This method, however, is not suitable to visualize endothelial barrier damage prior to microscopically detectable morphological alterations of the arterial wall such as sub-endothelial lipid deposition. To enable the investigation of early endothelial barrier damage and in particular the initial steps of atherosclerosis, a new method has to fulfill three requirements: (i) easy and fast to perform, (ii) low cost of applicability without requirement for highly sophisticated technical equipment, and (iii) reliable reproducibility of valid results. To this end, we used intracardial Evans blue dye injection after washout of blood and measured dye deposition within the aortic wall as a parameter of endothelial barrier leakiness, which is recognized as one of the earliest signs of atherosclerotic plaque formation. These analyses were performed in ApoE −/−, LDL receptor −/− and Cc1 −/− mouse models which have been reported to develop aortic plaques with or without high cholesterol diet. Our data show that sub-endothelial dye deposition is a reliable and reproducible readout parameter to assess endothelial barrier damage. Along these lines, measurements of aortic intima areas with Evans blue deposition in relation to total intima circumference enabled quantitative assessments of the results. Our technique enables the imaging of endothelial barrier damage prior to detectable aortic lipid deposition and plaque development. Thus, it will facilitate the detection of the initial vascular pathogenetic processes that lead to cardiovascular diseases. It will also enable the testing of new drugs and therapeutic procedures to prevent these disorders.







Similar content being viewed by others
References
Adamson RH, Curry FE, Adamson G, Liu B, Jiang Y, Aktories K, Barth H, Daigeler A, Golenhofen N, Ness W, Drenckhahn D (2002) Rho and rho kinase modulation of barrier properties: cultured endothelial cells and intact microvessels of rats and mice. J Physiol 539(Pt 1):295–308
Bell FP, Somer JB, Craig IH, Schwartz CJ (1972) Patterns of aortic Evans blue uptake in vivo and in vitro. Atherosclerosis 16(3):369–375
Benest AV, Kruse K, Savant S, Thomas M, Laib A, Loos EK, Fiedler U, Augustin HG (2013) Angiopoietin-2 is critical for cytokine-induced vascular leakage. PLoS ONE 8(8):e70459. doi:10.1371/journal.pone.0070459
Curry F-RE, Adamson RH (2010) Vascular permeability modulation at the cell, microvessel, or whole organ level: towards closing gaps in our knowledge. Cardiovasc Res 87(2):218–229. doi:10.1093/cvr/cvq115
Davignon J, Ganz P (2004) Role of endothelial dysfunction in atherosclerosis. Circulation 109 (23 Suppl 1):III27–III32. doi:10.1161/01.CIR.0000131515.03336.f8
Egawa G, Nakamizo S, Natsuaki Y, Doi H, Miyachi Y, Kabashima K (2013) Intravital analysis of vascular permeability in mice using two-photon microscopy. Sci Rep. doi:10.1038/srep01932
Gammal EB, Monture MC (1979) Uptake of Evans blue-bound albumin in the aorta of oestrogen-treated rats. Br J Exp Pathol 60(1):58–64
Gokce N, Keaney JF, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA (2003) Predictive value of noninvasivelydetermined endothelial dysfunction for long-term cardiovascular events inpatients with peripheral vascular disease. J Am Coll Cardiol 41(10):1769–1775. doi:10.1016/S0735-1097(03)00333-4
Gutierrez E, Flammer AJ, Lerman L, Elizaga J, Lerman A, Fernandez-Aviles F (2013) Endothelial dysfunction over the course of coronary artery disease. Eur Heart J 34(41):3175–3181. doi:10.1093/eurheartj/eht351
Hadi HA, Carr CS, Al SJ (2005) Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag 1(3):183–198
Heitzer T, Baldus S, Kodolitsch Y, Rudolph V, Meinertz T (2005) Systemic endothelial dysfunction as an early predictor of adverse outcome in heart failure. Arterioscler Thromb Vasc Biol 25(6):1174–1179. doi:10.1161/01.ATV.0000166516.52477.81
Higashi Y, Kihara Y, Noma K (2012) Endothelial dysfunction and hypertension in aging. Hypertens Res 35(11):1039–1047. doi:10.1038/hr.2012.138
Hou ST, Nilchi L, Li X, Gangaraju S, Jiang SX, Aylsworth A, Monette R, Slinn J (2015) Semaphorin3A elevates vascular permeability and contributes to cerebral ischemia-induced brain damage. Sci Rep 5:7890. doi:10.1038/srep07890
Jaipersad AS, Lip GYH, Silverman S, Shantsila E (2014) The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol 63(1):1–11. doi:10.1016/j.jacc.2013.09.019
Khan F, Galarraga B, Belch JJF (2010) The role of endothelial function and its assessment in rheumatoid arthritis. Nat Rev Rheumatol 6(5):253–261. doi:10.1038/nrrheum.2010.44
Leung N, Turbide C, Olson M, Marcus V, Jothy S, Beauchemin N (2006) Deletion of the carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1) gene contributes to colon tumor progression in a murine model of carcinogenesis. Oncogene 25(40):5527–5536. doi:10.1038/sj.onc.1209541
Libby P, Ridker PM, Hansson GK (2011) Progress and challenges in translating the biology of atherosclerosis. Nature 473(7347):317–325. doi:10.1038/nature10146
Lundeberg E, Van Der Does AM, Kenne E, Soehnlein O, Lindbom L (2015) Assessing large-vessel endothelial permeability using near-infrared fluorescence imaging-brief report. Arterioscler Thromb Vasc Biol 35(4):783–786. doi:10.1161/ATVBAHA.114.305131
Miles AA, Miles EM (1952) Vascular reactions to histamine, histamine-liberator and leukotaxine in the skin of guinea-pigs. J Physiol 118(2):228–257
Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, Zamorano JL, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Valgimigli M, Claeys MJ, Donner-Banzhoff N, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey, Hamilos M, Husted S, James SK, Kervinen K, Kristensen SD, Maggioni AP, Pries AR, Romeo F, Ryden L, Simoons ML, Steg PG, Timmis A, Yildirir A (2013) 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 34(38):2949–3003. doi:10.1093/eurheartj/eht296
Moore KJ, Sheedy FJ, Fisher EA (2013) Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 13(10):709–721. doi:10.1038/nri3520
Najjar SM, Ledford KJ, Abdallah SL, Paus A, Russo L, Kaw MK, Ramakrishnan SK, Muturi HT, Raphael CK, Lester SG, Heinrich G, Pierre SV, Benndorf R, Kleff V, Jaffa AA, Levy E, Vazquez G, Goldberg IJ, Beauchemin N, Scalia R, Ergun S (2013) Ceacam1 deletion causes vascular alterations in large vessels. Am J Physiol Endocrinol Metab 305(4):E519–E529. doi:10.1152/ajpendo.00266.2013
Nouvion A-L, Oubaha M, Leblanc S, Davis EC, Jastrow H, Kammerer R, Breton V, Turbide C, Ergun S, Gratton J-P, Beauchemin N (2010) CEACAM1: a key regulator of vascular permeability. J Cell Sci 123(Pt 24):4221–4230. doi:10.1242/jcs.073635
Radeva MY, Kugelmann D, Spindler V, Waschke J (2014) PKA compartmentalization via AKAP220 and AKAP12 contributes to endothelial barrier regulation. PLoS ONE 9(9):e106733. doi:10.1371/journal.pone.0106733
Simoneau B, Houle F, Huot J (2012) Regulation of endothelial permeability and transendothelial migration of cancer cells by tropomyosin-1 phosphorylation. Vasc Cell 4(1):18. doi:10.1186/2045-824X-4-18
Singh NK, Kotla S, Dyukova E, Traylor JG, Orr AW, Chernoff J, Marion TN, Rao GN (2015) Disruption of p21-activated kinase 1 gene diminishes atherosclerosis in apolipoprotein E-deficient mice. Nat Commun 6:7450. doi:10.1038/ncomms8450
Sitia S, Tomasoni L, Atzeni F, Ambrosio G, Cordiano C, Catapano A, Tramontana S, Perticone F, Naccarato P, Camici P, Picano E, Cortigiani L, Bevilacqua M, Milazzo L, Cusi D, Barlassina C, Sarzi-Puttini P, Turiel M (2010) From endothelial dysfunction to atherosclerosis. Autoimmun Rev 9(12):830–834. doi:10.1016/j.autrev.2010.07.016
Sjoland H, Eitzman DT, Gordon D, Westrick R, Nabel EG, Ginsburg D (2000) Atherosclerosis progression in LDL receptor-deficient and apolipoprotein E-deficient mice is independent of genetic alterations in plasminogen activator inhibitor-1. Arterioscler Thromb Vasc Biol 20(3):846–852
Srinivasan B, Kolli AR, Esch MB, Abaci HE, Shuler ML, Hickman JJ (2015) TEER measurement techniques for in vitro barrier model systems. J Lab Autom 20(2):107–126. doi:10.1177/2211068214561025
Tabas I, Garcia-Cardena G, Owens GK (2015) Recent insights into the cellular biology of atherosclerosis. J Cell Biol 209(1):13–22. doi:10.1083/jcb.201412052
Wang W, Lee Y, Lee CH (2013) Review: the physiological and computational approaches for atherosclerosis treatment. Int J Cardiol 167(5):1664–1676. doi:10.1016/j.ijcard.2012.09.195
Weber C, Noels H (2011) Atherosclerosis: current pathogenesis and therapeutic options. Nat Med 17(11):1410–1422. doi:10.1038/nm.2538
Whitman SC (2004) A practical approach to using mice in atherosclerosis research. Clin Biochem Rev 25 (1):81–93
Wiltshire R, Nelson V, Kho DT, Angel CE, O’Carroll SJ, Graham ES (2016) Regulation of human cerebro-microvascular endothelial baso-lateral adhesion and barrier function by S1P through dual involvement of S1P1 and S1P2 receptors. Sci Rep 6:19814. doi:10.1038/srep19814
Woollard KJ, Geissmann F (2010) Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 7(2):77–86. doi:10.1038/nrcardio.2009.228
Acknowledgements
The authors are grateful to Tanja Metzger for excellent technical assistance. We thank the Deutsche Forschungsgemeinschaft (DFG) for the financial support by the Collaborative Research Center SFB688 TP A19 and TP A22.
Author contributions
H.B. and F.K. designed and carried out experiments, analyzed the data and wrote the manuscript. A.Z. interpreted the results and provided financial support for the research. S.G. and N.W. carried out experiments. S.K. interpreted results and wrote the manuscript. S.E. directed the study, analyzed the data, wrote the manuscript and provided financial support for the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Tissue probes from experimental animals were performed in accordance with the Helsinki Declaration and German law (Tierschutzgesetz BGBl. I,S. 1206, Revision 2006).
Additional information
Heike Bömmel and Florian Kleefeldt have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Bömmel, H., Kleefeldt, F., Zernecke, A. et al. Visualization of endothelial barrier damage prior to formation of atherosclerotic plaques. Histochem Cell Biol 148, 117–127 (2017). https://doi.org/10.1007/s00418-017-1562-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-017-1562-8