Abstract
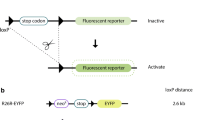
Our understanding about medullary compartment, its niches composition and formation is still limited. Previous studies using EphB2 and/or EphB3 knockout mice showed an abnormal thymic development that affects mainly to the epithelial component, including the cortex/medulla distribution, thymic epithelial cell (TEC) morphology and different epithelial-specific marker expression. We have already demonstrated that the lack of ephrinB1 and/or ephrinB2, either on thymocytes or on TECs, alters the cell intermingling processes necessary for thymus organization and affect cortical TEC subpopulations. In the present work, we have used the Cre–LoxP model to selectively delete ephrinB1 and/or ephrinB2 in thymocytes (EfnB1thy/thy, EfnB2thy/thy, EfnB1thy/thyEfnB2thy/thy mice) or TECs (EfnB1tec/tec, EfnB2tec/tec, EfnB1tec/tecEfnB2tec/tec mice) and have analyzed their role on the medullary compartment. In all the studied mutants, medullary areas are smaller and more compact than in the wt thymuses. In most of them, we observe abundant big cysts and a higher proportion of UEAhiMTS10− cells than in wt mice, which are often forming small cysts. On EfnB1tec/tecEfnB2tec/tec, changes affecting organ size and medullary compartment start at perinatal stage. Our data shed some light on knowledge about wt medulla histological structure and cysts meaning and formation process and on the role played by ephrinB in them.






Similar content being viewed by others
References
Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM, Matsumoto M, Nitta T, Takahama Y, Inoue J (2008) The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity 29:423–437
Akiyama T, Shinzawa M, Akiyama N (2012) TNF receptor family signaling in the development and functions of medullary thymic epithelial cells. Front Immunol 3:278
Alfaro D, Munoz JJ, Garcia-Ceca J, Cejalvo T, Jimenez E, Zapata AG (2011) The Eph/ephrinB signal balance determines the pattern of T-cell maturation in the thymus. Immunol Cell Biol 89:844–852
Alves NL, Takahama Y, Ohigashi I, Ribeiro AR, Baik S, Anderson G, Jenkinson WE (2014) Serial progression of cortical and medullary thymic epithelial microenvironments. Eur J Immunol 44:16–22
Anderson G, Takahama Y (2012) Thymic epithelial cells: working class heroes for T cell development and repertoire selection. Trends Immunol 33:256–263
Anderson G, Baik S, Cowan JE, Holland AM, McCarthy NI, Nakamura K, Parnell SM, White AJ, Lane PJ, Jenkinson EJ, Jenkinson WE (2014) Mechanisms of thymus medulla development and function. Curr Top Microbiol Immunol 373:19–47
Baik S, Jenkinson EJ, Lane PJ, Anderson G, Jenkinson WE (2013) Generation of both cortical and Aire(+) medullary thymic epithelial compartments from CD205(+) progenitors. Eur J Immunol 43:589–594
Benz C, Heinzel K, Bleul CC (2004) Homing of immature thymocytes to the subcapsular microenvironment within the thymus is not an absolute requirement for T cell development. Eur J Immunol 34:3652–3663
Calderon L, Boehm T (2012) Synergistic, context-dependent, and hierarchical functions of epithelial components in thymic microenvironments. Cell 149:159–172
Cejalvo T, Munoz JJ, Tobajas E, Fanlo L, Alfaro D, Garcia-Ceca J, Zapata A (2013) Ephrin-B-dependent thymic epithelial cell-thymocyte interactions are necessary for correct T cell differentiation and thymus histology organization: relevance for thymic cortex development. J Immunol 190:2670–2681
Coquet JM, Ribot JC, Babala N, Middendorp S, van der Horst G, Xiao Y, Neves JF, Fonseca-Pereira D, Jacobs H, Pennington DJ, Silva-Santos B, Borst J (2013) Epithelial and dendritic cells in the thymic medulla promote CD4 + Foxp3 + regulatory T cell development via the CD27-CD70 pathway. J Exp Med 210:715–728
Davy A, Aubin J, Soriano P (2004) Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev 18:572–583
Desanti GE, Cowan JE, Baik S, Parnell SM, White AJ, Penninger JM, Lane PJ, Jenkinson EJ, Jenkinson WE, Anderson G (2012) Developmentally regulated availability of RANKL and CD40 ligand reveals distinct mechanisms of fetal and adult cross-talk in the thymus medulla. J Immunol 189:5519–5526
Farr AG, Dooley JL, Erickson M (2002) Organization of thymic medullary epithelial heterogeneity: implications for mechanisms of epithelial differentiation. Immunol Rev 189:20–27
Fiala JC (2005) Reconstruct: a free editor for serial section microscopy. J Microsc 218:52–61
Garcia-Ceca J, Jimenez E, Alfaro D, Cejalvo T, Chumley MJ, Henkemeyer M, Munoz JJ, Zapata AG (2009a) On the role of Eph signalling in thymus histogenesis; EphB2/B3 and the organizing of the thymic epithelial network. Int J Dev Biol 53:971–982
Garcia-Ceca J, Jimenez E, Alfaro D, Cejalvo T, Munoz JJ, Zapata AG (2009b) Cell-autonomous role of EphB2 and EphB3 receptors in the thymic epithelial cell organization. Eur J Immunol 39:2916–2924
Germeraad WT, Kawamoto H, Itoi M, Jiang Y, Amagai T, Katsura Y, van Ewijk W (2003) Development of thymic microenvironments in vitro is oxygen-dependent and requires permanent presence of T-cell progenitors. J Histochem Cytochem 51:1225–1235
Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM, Goodnow CC, Boyd RL (2006) Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood 108:3777–3785
Guo J, Rahman M, Cheng L, Zhang S, Tvinnereim A, Su DM (2011) Morphogenesis and maintenance of the 3D thymic medulla and prevention of nude skin phenotype require FoxN1 in pre- and post-natal K14 epithelium. J Mol Med 89(3):263–277
Hamazaki Y, Fujita H, Kobayashi T, Choi Y, Scott HS, Matsumoto M, Minato N (2007) Medullary thymic epithelial cells expressing Aire represent a unique lineage derived from cells expressing claudin. Nat Immunol 8:304–311
Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H, Yokota Y, Yamada H, Yoshikai Y, Inoue J, Akiyama T, Takahama Y (2008) The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity 29:438–450
Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, Hubert FX, Scott HS, Takahama Y, Hollander GA, Reith W (2008) Autoantigen-specific interactions with CD4 + thymocytes control mature medullary thymic epithelial cell cellularity. Immunity 29:451–463
Jin W, Qi S, Luo H (2011) The effect of conditional EFNB1 deletion in the T cell compartment on T cell development and function. BMC Immunol 12:68
Jin W, Qi S, Luo H (2012) T cell-specific deletion of EFNB2 minimally affects T cell development and function. Mol Immunol 52:141–147
Jin W, Luo H, Wu J (2014) Effect of reduced EPHB4 expression in thymic epithelial cells on thymocyte development and peripheral T cell function. Mol Immunol 58:1–9
Klug DB, Carter C, Gimenez-Conti IB, Richie ER (2002) Cutting edge: thymocyte-independent and thymocyte-dependent phases of epithelial patterning in the fetal thymus. J Immunol 169:2842–2845
Luo H, Yu G, Tremblay J, Wu J (2004) EphB6-null mutation results in compromised T cell function. J Clin Invest 114:1762–1773
Luo H, Wu Z, Qi S, Jin W, Han B, Wu J (2011) Ephrinb1 and Ephrinb2 are associated with interleukin-7 receptor alpha and retard its internalization from the cell surface. J Biol Chem 286:44976–44987
Munoz JJ, Alfaro D, Garcia-Ceca J, Alonso CL, Jimenez E, Zapata A (2006) Thymic alterations in EphA4-deficient mice. J Immunol 177:804–813
Munoz JJ, Cejalvo T, Alonso-Colmenar LM, Alfaro D, Garcia-Ceca J, Zapata A (2011) Eph/Ephrin-mediated interactions in the thymus. Neuroimmunomodulation 18:271–280
Ohigashi I, Zuklys S, Sakata M, Mayer CE, Zhanybekova S, Murata S, Tanaka K, Hollander GA, Takahama Y (2013) Aire-expressing thymic medullary epithelial cells originate from beta5t-expressing progenitor cells. Proc Natl Acad Sci USA 110:9885–9890
Osada M, Ito E, Fermin HA, Vazquez-Cintron E, Venkatesh T, Friedel RH, Pezzano M (2006) The Wnt signaling antagonist Kremen1 is required for development of thymic architecture. Clin Dev Immunol 13:299–319
Palmer DB, Viney JL, Ritter MA, Hayday AC, Owen MJ (1993) Expression of the alpha beta T-cell receptor is necessary for the generation of the thymic medulla. Dev Immunol 3:175–179
Pasquale EB (2005) Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol 6:462–475
Poliakov A, Cotrina M, Wilkinson DG (2004) Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell 7:465–480
Revest JM, Suniara RK, Kerr K, Owen JJ, Dickson C (2001) Development of the thymus requires signaling through the fibroblast growth factor receptor R2-IIIb. J Immunol 167:1954–1961
Ribeiro AR, Rodrigues PM, Meireles C, Di Santo JP, Alves NL (2014) Thymocyte selection regulates the homeostasis of IL-7-expressing thymic cortical epithelial cells in vivo. J Immunol 191:1200–1209
Rossi SW, Chidgey AP, Parnell SM, Jenkinson WE, Scott HS, Boyd RL, Jenkinson EJ, Anderson G (2007) Redefining epithelial progenitor potential in the developing thymus. Eur J Immunol 37:2411–2418
Sano S, Takahama Y, Sugawara T, Kosaka H, Itami S, Yoshikawa K, Miyazaki J, van Ewijk W, Takeda J (2001) Stat3 in thymic epithelial cells is essential for postnatal maintenance of thymic architecture and thymocyte survival. Immunity 15:261–273
Shimizu C, Kawamoto H, Yamashita M, Kimura M, Kondou E, Kaneko Y, Okada S, Tokuhisa T, Yokoyama M, Taniguchi M, Katsura Y, Nakayama T (2001) Progression of T cell lineage restriction in the earliest subpopulation of murine adult thymus visualized by the expression of lck proximal promoter activity. Int Immunol 13:105–117
Shores EW, Van Ewijk W, Singer A (1994) Maturation of medullary thymic epithelium requires thymocytes expressing fully assembled CD3-TCR complexes. Int Immunol 6:1393–1402
van Ewijk W, Wang B, Hollander G, Kawamoto H, Spanopoulou E, Itoi M, Amagai T, Jiang YF, Germeraad WT, Chen WF, Katsura Y (1999) Thymic microenvironments, 3-D versus 2-D? Semin Immunol 11:57–64
Vroegindeweij E, Crobach S, Itoi M, Satoh R, Zuklys S, Happe C, Germeraad WT, Cornelissen JJ, Cupedo T, Hollander GA, Kawamoto H, van Ewijk W (2010) Thymic cysts originate from Foxn1 positive thymic medullary epithelium. Mol Immunol 47:1106–1113
White AJ, Nakamura K, Jenkinson WE, Saini M, Sinclair C, Seddon B, Narendran P, Pfeffer K, Nitta T, Takahama Y, Caamano JH, Lane PJ, Jenkinson EJ, Anderson G (2010) Lymphotoxin signals from positively selected thymocytes regulate the terminal differentiation of medullary thymic epithelial cells. J Immunol 185:4769–4776
Acknowledgments
This work was supported by grants BFU2007-65520 and BFU2010-18250 from Spanish Ministry of Education and Science; RD06/0010/0003 and RD12/0019/0007 from Spanish Ministry of Health and Consume, and S-BIO/0204/2006 and R74/91-05552/08 from the Regional Government of Madrid. We thank Dr. D.J. Anderson (California Institute of Technology, Pasadena, California) for providing C57BL/6-EfnB1LoxP/LoxP mice, Dr. J.J. Takeda and the Center for Animal Resources and Development of the Kumamoto University (Japan) for the gift of C57BL/6-Tg(K5-Cre) Jt strain and Dr. R. Boyd, (Monash University, Melbourne, Australia) for the donation of the MTS10 and MTS20 antibodies. We would like to also thank to the “Developmental Studies Hybridoma Bank” of Iowa University for supplying the anti-K8 keratin antibody and to the Cytometry and Fluorescence Microscopy Centre at Complutense University of Madrid for the use of its facilities and technical assistance.
Conflict of interest
The authors declare no commercial or financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Teresa Cejalvo and Juan J. Munoz have contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cejalvo, T., Munoz, J.J., Tobajas, E. et al. Conditioned deletion of ephrinB1 and/or ephrinB2 in either thymocytes or thymic epithelial cells alters the organization of thymic medulla and favors the appearance of thymic epithelial cysts. Histochem Cell Biol 143, 517–529 (2015). https://doi.org/10.1007/s00418-014-1296-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-014-1296-9