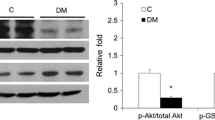
Abstract
Diabetic nephropathy (DN) is a major complication of diabetic patients and the leading cause of end-stage renal disease. Glomerular dysfunction plays a critical role in DN, but deterioration of renal function also correlates with tubular alterations. Human DN is characterized by glycogen accumulation in tubules. Although this pathological feature has long been recognized, little information exists about the triggering mechanism. In this study, we detected over-expression of muscle glycogen synthase (MGS) in diabetic human kidney. This enhanced expression suggests the participation of MGS in renal metabolic changes associated with diabetes. HK2 human renal cell line exhibited an intrinsic ability to synthesize glycogen, which was enhanced after over-expression of protein targeting to glycogen. A correlation between increased glycogen amount and cell death was observed. Based on a previous transcriptome study on human diabetic kidney disease, significant differences in the expression of genes involved in glycogen metabolism were analyzed. We propose that glucose, but not insulin, is the main modulator of MGS activity in HK2 cells, suggesting that blood glucose control is the best approach to modulate renal glycogen-induced damage during long-term diabetes.





Similar content being viewed by others
References
Armanni L (1877) Fünf autopsien mit histologischen untersuchungen und klinischer epicrise. In: Cantani A (ed) Diabetes mellitus. Berlin, Germany, Vierzehnte Vorlesung
Baba O (1993) Production of monoclonal antibody that recognizes glycogen and its application for immunohistochemistry. Kokubyo Gakkai Zasshi 60(2):264–287
Bamri-Ezzine S, Ao ZJ, Londoño I, Gingras D, Bendayan M (2003) Apoptosis of tubular epithelial cells in glycogen nephrosis during diabetes. Lab Invest 83(7):1069–1080
Berry GT, Baker L, Kaplan FS, Witzleben CL (1995) Diabetes-like renal glomerular disease in Fanconi–Bickel syndrome. J Pediatr Nephrol 9(3):287–291
Biava C, Grossman A, West M (1966) Ultrastructural observations on renal glycogen in normal and pathological human kidneys. Lab Invest 15(1 Pt 2):330–356
Bobulescu IA, Dubree M, Zhang J, McLeroy P, Moe OW (2009) Reduction of renal triglyceride accumulation: effects on proximal tubule Na+/H+ exchange and urinary acidification. Am J Physiol Renal Physiol 297:F1419–F1426
Bollen M (2001) Combinatorial control of protein phosphatase-1. Trends Biochem Sci 26(7):426–431
Cammisotto PG, Londono I, Gingras D, Bendayan M (2008) Control of glycogen synthase through ADIPOR1-AMPK pathway in renal distal tubules of normal and diabetic rats. Am J Physiol Renal Physiol 294(4):F881–F889
Curtis GW, Robbins SL, Glickman I (1947) Studies on glycogen nephrosis in alloxan-treated diabetic rats. J Exp Med 85(4):373–379
DeFronzo RA, Davidson JA, Del Prato S (2012) The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 14(1):5–14
Dombrowski D, Klotz L, Bannasch P, Evert M (2007) Renal carcinogenesis in models of diabetes in rats—metabolic changes are closely related to neoplastic development. Diabetologia 50:2580–2590
Duran J, Tevy MF, García-Rocha M, Calbó J, Milán M, Guinovart JJ (2012) Deleterious effects of neuronal accumulation of glycogen in flies and mice. EMBO Mol Med 8:719–729
Eckardt KU, Bernhardt WM, Weidemann A, Warnecke C, Rosenberger C, Wiesener MS, William C (2005) Role of hypoxia in the pathogenesis of renal disease. Kidney Int Suppl 99:S46–S51
Ferrer JC, Favre C, Gomis RR, Fernández-Novell JM, García-Rocha M, de la Iglesia N, Cid E, Guinovart JJ (2003) Control of glycogen deposition. FEBS Lett 546:127–132
Gatica R, Bertinat R, Silva P, Carpio D, Ramírez MJ, Slebe JC, San Martín R, Nualart F, Campistol JM, Caelles C, Yáñez AJ (2013) Altered expression and localization of insulin receptor in proximal tubule cells from human and rat diabetic kidney. J Cell Biochem 114:639–649
Graham TE (2009) Glycogen: an overview of possible regulatory roles of the proteins associated with the granule. Appl Physiol Nutr Metab 34(3):488–492
Højlund K, Stæhr P, Hansen BF, Green KA, Hardie DG, Richter EA, Beck-Nielsen H, Wojtaszewski JF (2003) Increased phosphorylation of skeletal muscle glycogen synthase at NH2-terminal sites during physiological hyperinsulinemia in type 2 diabetes. Diabetes 52(6):1393–1402
Holck P, Rasch R (1993) Structure and segmental localization of glycogen in the diabetic rat kidney. Diabetes 42(6):891–900
Jørgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, Schjerling P, Vaulont S, Hardie DG, Hansen BF, Richter EA, Wojtaszewski JF (2004) The α2–5′AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes 53:3074–3081
Kang J, Dai XS, Yu TB, Wen B, Yang ZW (2005) Glycogen accumulation in renal tubules, a key morphological change in the diabetic rat kidney. Acta Diabetol 42(2):110–116
Kaslow HR, Lesikar DD (1984) Isozymes of glycogen synthase. FEBS Lett 172(2):294–298
Kaslow HR, Lesikar DD, Antwi D, Tan AW (1985) L-type glycogen synthase. Tissue distribution and electrophoretic mobility. J Biol Chem 260(18):9953–9956
Khandelwal RL, Zinman SM, Knull HR (1979) The effect of streptozotocin-induced diabetes on glycogen metabolism in rat kidney and its relationship to the liver system. Arch Biochem Biophys 197(1):310–316
Kugler JH, Wilkinson WJ (1964) Quantitative studies with the solutions used for the fixation of glycogen. Acta Anat (Basel) 56:184–195
Kume S, Thomas MC, Koya D (2012) Nutrient sensing, autophagy, and diabetic nephropathy. Diabetes 61(1):23–29
Linden KC, DeHaan CL, Zhang Y, Glowacka S, Cox AJ, Kelly DJ, Rogers S (2006) Renal expression and localization of the facilitative glucose transporters GLUT1 and GLUT12 in animal models of hypertension and diabetic nephropathy. Am J Physiol Renal Physiol 290:F205–F213
MacAulay K, Blair AS, Hajduch E, Terashima T, Baba O, Sutherland C, Hundal HS (2005) Constitutive activation of GSK3 down-regulates glycogen synthase abundance and glycogen deposition in rat skeletal muscle cells. J Biol Chem 280(10):9509–9518
Marks J, Carvou NJC, Debnam ES, Srai SK, Unwin RJ (2003) Diabetes increases facilitative glucose uptake and GLUT2 expression at the rat proximal tubule brush border membrane. J Physiol 553(1):137–145
Meyer C, Woerle HJ, Dostou JM, Welle SL, Gerich JE (2004) Abnormal renal, hepatic, and muscle glucose metabolism following glucose ingestion in type 2 diabetes. Am J Physiol Endocrinol Metab 287(6):E1049–E1056
Nannipieri M, Lanfranchi A, Santerini D, Catalano C, Van de Werve G, Ferrannini E (2001) Influence of long-term diabetes on renal glycogen metabolism in the rat. Nephron 87(1):50–57
Palm F (2006) Intrarenal oxygen in diabetes and a possible link to diabetic nephropathy. Clin Exp Pharmacol Physiol 33(10):997–1001
Pescador N, Villar D, Cifuentes D, García-Rocha M, Ortiz-Barahona A, Vázquez S, Ordoñez A, Cuevas Y, Sáez-Morales D, García-Bermejo ML, Landazuri MO, Guinovart J, del Peso L (2010) Hypoxia promotes glycogen accumulation through hypoxia inducible factor (HIF)-mediated induction of glycogen synthase 1. PLoS ONE 5(3):e9644
Prats C, Helge JW, Nordby P, Qvortrup K, Ploug T, Dela F, Wojtaszewski JFP (2009) Dual regulation of muscle glycogen synthase during exercise by activation and compartmentalization. J Biol Chem 284(23):15692–15700
Prats C, Gómez-Cabello A, Nordby P, Andersen JL, Helge JW, Dela F, Baba O, Ploug T (2013) An optimized histochemical method to asses skeletal muscle glycogen and lipid stores reveals two metabolically distinct populations of type I muscle fibers. PLoS ONE 8(10):e77774
Reutens AT, Atkins RC (2011) Epidemiology of diabetic nephropathy. Contrib Nephrol 170:1–7
Ritchie S, Waugh D (1957) The pathology of Armanni-Ebstein diabetic nephropathy. Am J Pathol 33(6):1035–1057
Ros S, García-Rocha M, Domínguez J, Ferrer JC, Guinovart JJ (2009) Control of liver glycogen synthase activity and intracellular distribution by phosphorylation. J Biol Chem 284(10):6370–6378
Ros S, García-Rocha M, Calbó J, Guinovart JJ (2011) Restoration of hepatic glycogen deposition reduces hyperglycaemia, hyperphagia and gluconeogenic enzymes in a streptozotocin-induced model of diabetes in rats. Diabetologia 54(10):2639–2648
Sakamaki J, Daitoku H, Kaneko Y, Hagiwara A, Ueno K, Fukamizu A (2012) GSK3β regulates gluconeogenic gene expression through HNF4α and FOXO1. J Recept Signal Transduct Res 32(2):96–101
Silva FG (2005) Diabetic nephropathy. In: D’Agati VD, Jennette JC, Silva FG (eds) Non-neoplastic kidney diseases. American Registry of Pathology, Washington, DC, pp 457–459
Singh DK, Winocour P, Farrington K (2008) Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat Clin Pract Nephrol 4(4):216–226
Trott JR (1961) An evaluation of methods commonly used for the fixation and staining of glycogen. J Histochem Cytochem 9:703–710
Vallon V (2011) The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 300:1009–1022
Vestergaard H, Lund S, Bjorbaek C, Pedersen O (1995) Unchanged gene expression of glycogen synthase in muscle from patients with NIDDM following sulphonylurea-induced improvement of glycaemic control. Diabetologia 38(10):1230–1238
Vigoda A, Mamedova LK, Shneyvays V, Katz A, Shainberg A (2003) Glycogen metabolism in rat heart muscle cultures after hypoxia. Mol Cell Biochem 254(1–2):311–318
Vilchez D, Ros S, Cifuentes D, Pujadas L, Valles J, García-Fojeda B, Criado-García O, Fernández-Sánchez E, Medraño-Fernández I, Domínguez J, García-Rocha M, Soriano E, Rodríguez de Córdoba S, Guinovart JJ (2007) Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat Neurosci 10(11):1407–1413
Villarroel-Espíndola F, Maldonado R, Mancilla H, vander Stelt K, Acuña AI, Covarrubias A, López C, Angulo C, Castro MA, Slebe JC, Durán J, García-Rocha M, Guinovart JJ, Concha II (2013) Muscle glycogen synthase isoform is responsible for testicular glycogen synthesis: glycogen overproduction induces apoptosis in male germ cells. J Cell Biochem 114(7):1653–1664
Wilson-O’Brien AL, Patron N, Rogers S (2010) Evolutionary ancestry and novel functions of the mammalian glucose transporter (GLUT) family. BMC Evol Biol 10:152
Wirthensohn G, Guder WG (1986) Renal substrate metabolism. Physiol Rev 66(2):469–497
Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K (2011) Transcriptome analysis of human diabetic kidney disease. Diabetes 60(9):2354–2369
Yáñez AJ, Ludwig HC, Bertinat R, Spichiger C, Gatica R, Berlien G, Leon O, Brito M, Concha II, Slebe JC (2005) Different involvement for aldolase isoenzymes in kidney glucose metabolism: aldolase B but not aldolase A colocalizes and forms a complex with FBPase. J Cell Physiol 202:743–753
Young FG (1957) Claude bernard and the discovery of glycogen; a century of retrospect. Br Med J 1(5033):1431–1437
Zakout YM, Salih MM, Ahmed HG (2010) The effect of fixatives and temperature on the quality of glycogen demonstration. Biotech Histochem 85(2):93–98 (also see Erratum, Biotech Histochem (2011) 86(6):448)
Acknowledgments
The authors thank Dr. Ilona Concha and Elizabeth Mann for correcting the English manuscript. Microscopy analysis was performed at Centro de Microscopía Avanzada (CMA)-Bio Bio, Universidad de Concepción, Concepción, Chile. This study was supported by grants from Fondo Nacional de Desarrollo Científico y Tecnológico from Chilean State: FONDECYT 1131033 to A. Yañez; FONDECYT 3120144 to R. Bertinat.
Conflict of interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Rodrigo Gatica and Romina Bertinat have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gatica, R., Bertinat, R., Silva, P. et al. Over-expression of muscle glycogen synthase in human diabetic nephropathy. Histochem Cell Biol 143, 313–324 (2015). https://doi.org/10.1007/s00418-014-1290-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-014-1290-2