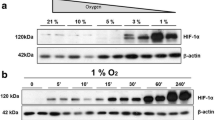
Abstract
Binding of endothelial cell (EC) integrins to extracellular-matrix (ECM) components is one of the key events to trigger intracellular signaling that will ultimately result in proper vascular development. Even within one tissue, the endothelial phenotype differs between arteries and veins. Here, we tested the hypothesis that anchorage-dependent processes, such as proliferation, viability, survival and actin organization of venous (VEC) and arterial EC (AEC) differently depend on ECM proteins. Moreover, because of different oxygen tension in AEC and VEC, we tested oxygen as a co-modulator of ECM effects. Primary human placental VEC and AEC were grown in collagens I and IV, fibronectin, laminin, gelatin and uncoated plates and exposed to 12 and 21% oxygen. Our main findings revealed that VEC are more sensitive than AEC to changes in the ECM composition. Proliferation and survival of VEC, in contrast to AEC, were profoundly increased by the presence of collagen I and fibronectin when compared with gelatin or uncoated plates. These effects were reversed by inhibition of focal adhesion kinase (Fak) and modulated by oxygen. VEC were more susceptible to the oxygen-dependent ECM effects than AEC. However, no differential ECM effect on actin organization was observed between the two cell types. These data provide first evidence that AEC and VEC from the same vascular loop respond differently to ECM and oxygen in a Fak-dependent manner.






Similar content being viewed by others
References
Adachi T, Wang X, Murata T, Obara M, Akutsu H, Machida M et al (2010) Production of a non-triple helical collagen alpha chain in transgenic silkworms and its evaluation as a gelatin substitute for cell culture. Biotechnol Bioeng 106:860–870
Alam N, Goel HL, Zarif MJ, Butterfield JE, Perkins HM, Sansoucy BG et al (2007) The integrin-growth factor receptor duet. J Cell Physiol 213:649–653
Amenta PS, Gay S, Vaheri A, Martinez-Hernandez A (1986) The extracellular matrix is an integrated unit: ultrastructural localization of collagen types I, III, IV, V, VI, fibronectin, and laminin in human term placenta. Coll Relat Res 6:125–152
Annas A, Granberg AL, Brittebo EB (2000) Differential response of cultured human umbilical vein and artery endothelial cells to Ah receptor agonist treatment: CYP-dependent activation of food and environmental mutagens. Toxicol Appl Pharmacol 169:94–101
Arnaoutova I, George J, Kleinman HK, Benton G (2009) The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis 12:267–274
Aspenstrom P (1999) Effectors for the Rho GTPases. Curr Opin Cell Biol 11:95–102
Beierle EA, Ma X, Trujillo A, Kurenova EV, Cance WG, Golubovskaya VM (2010) Inhibition of focal adhesion kinase and src increases detachment and apoptosis in human neuroblastoma cell lines. Mol Carcinog 49:224–234
Bill HM, Knudsen B, Moores SL, Muthuswamy SK, Rao VR, Brugge JS et al (2004) Epidermal growth factor receptor-dependent regulation of integrin-mediated signaling and cell cycle entry in epithelial cells. Mol Cell Biol 24:8586–8599
Blaschitz A, Weiss U, Dohr G, Desoye G (2000) Antibody reaction patterns in first trimester placenta: implications for trophoblast isolation and purity screening. Placenta 21:733–741
Borm B, Requardt RP, Herzog V, Kirfel G (2005) Membrane ruffles in cell migration: indicators of inefficient lamellipodia adhesion and compartments of actin filament reorganization. Exp Cell Res 302:83–95
Boudreau NJ, Jones PL (1999) Extracellular matrix and integrin signalling: the shape of things to come. Biochem J 339(Pt 3):481–488
Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G et al (1994) Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 79:1157–1164
Cabodi S, Di Stefano P, Leal Mdel P, Tinnirello A, Bisaro B, Morello V et al (2010) Integrins and signal transduction. Adv Exp Med Biol 674:43–54
Cervar M, Blaschitz A, Dohr G, Desoye G (1999) Paracrine regulation of distinct trophoblast functions in vitro by placental macrophages. Cell Tissue Res 295:297–305
Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S et al (1999) Mechanotransduction in response to shear stress roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem 274:18393–18400
Chen CM, Wang LF, Chou HC, Lang YD, Lai YP (2007) Up-regulation of connective tissue growth factor in hyperoxia-induced lung fibrosis. Pediatr Res 62:128–133
Chiarugi P (2008) From anchorage dependent proliferation to survival: lessons from redox signalling. IUBMB Life 60:301–307
Clark EA, King WG, Brugge JS, Symons M, Hynes RO (1998) Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol 142:573–586
Corley KM, Taylor CJ, Lilly B (2005) Hypoxia-inducible factor 1alpha modulates adhesion, migration, and FAK phosphorylation in vascular smooth muscle cells. J Cell Biochem 96:971–985
Falkenhain ALT, Behrendt U, Lehmann L (2002) Dead cell estimation—a comparison of different methods, new developments and new applications in animal cell technology. Kluwer, Dordrecht
Frisch SM, Screaton RA (2001) Anoikis mechanisms. Curr Opin Cell Biol 13:555–562
Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY (1996) Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol 134:793–799
Gao B, Curtis TM, Blumenstock FA, Minnear FL, Saba TM (2000) Increased recycling of (alpha)5(beta)1 integrins by lung endothelial cells in response to tumor necrosis factor. J Cell Sci 113(Pt 2):247–257
Golubovskaya VM, Nyberg C, Zheng M, Kweh F, Magis A, Ostrov D et al (2008) A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the y397 site of focal adhesion kinase decreases tumor growth. J Med Chem 51:7405–7416
Guan JL, Trevithick JE, Hynes RO (1991) Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul 2:951–964
Hanks SK, Calalb MB, Harper MC, Patel SK (1992) Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci USA 89:8487–8491
Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB (2005) Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 4:988–1004
Herr F, Baal N, Widmer-Teske R, McKinnon T, Zygmunt M (2010) How to study placental vascular development? Theriogenology 73:817–827
Hiden U, Glitzner E, Hartmann M, Desoye G (2009) Insulin and the IGF system in the human placenta of normal and diabetic pregnancies. J Anat 215:60–68
Hodivala-Dilke KM, Reynolds AR, Reynolds LE (2003) Integrins in angiogenesis: multitalented molecules in a balancing act. Cell Tissue Res 314:131–144
Honore S, Pichard V, Penel C, Rigot V, Prev tC, Marvaldi J et al (2000) Outside-in regulation of integrin clustering processes by ECM components per se and their involvement in actin cytoskeleton organization in a colon adenocarcinoma cell line. Histochem Cell Biol 114:323–335
Hood JD, Frausto R, Kiosses WB, Schwartz MA, Cheresh DA (2003) Differential alphav integrin-mediated Ras-ERK signaling during two pathways of angiogenesis. J Cell Biol 162:933–943
Hughes P, Marshall D, Reid Y, Parkes H, Gelber C (2007) The costs of using unauthenticated, over-passaged cell lines: how much more data do we need? Biotechniques 43:575, 577–578, 581–572, passim
Huppertz B, Kertschanska S, Frank HG, Gaus G, Funayama H, Kaufmann P (1996) Extracellular matrix components of the placental extravillous trophoblast: immunocytochemistry and ultrastructural distribution. Histochem Cell Biol 106:291–301
Hynes RO (2009) The extracellular matrix: not just pretty fibrils. Science 326:1216–1219
Jean C, Gravelle P, Fournie JJ, Laurent G (2011) Influence of stress on extracellular matrix and integrin biology. Oncogene 30:2697–2706
Jones PL, Crack J, Rabinovitch M (1997) Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the alpha v beta 3 integrin to promote epidermal growth factor receptor phosphorylation and growth. J Cell Biol 139:279–293
Juliano R (1996) Cooperation between soluble factors and integrin-mediated cell anchorage in the control of cell growth and differentiation. Bioessays 18:911–917
Kim SH, Turnbull J, Guimond S (2011) Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol 209:139–151
Kojima N, Sato M, Imai K, Miura M, Matano Y, Senoo H (1998) Hepatic stellate cells (vitamin A-storing cells) change their cytoskeleton structure by extracellular matrix components through a signal transduction system. Histochem Cell Biol 110:121–128
Korhonen M, Virtanen I (1997) The distribution of laminins and fibronectins is modulated during extravillous trophoblastic cell differentiation and decidual cell response to invasion in the human placenta. J Histochem Cytochem 45:569–581
Kuehn K, Schoene H-H, Timpl R (eds) (1982) New trends in basement membrane research. Raven Press, New York
Lang I, Pabst MA, Hiden U, Blaschitz A, Dohr G, Hahn T et al (2003) Heterogeneity of microvascular endothelial cells isolated from human term placenta and macrovascular umbilical vein endothelial cells. Eur J Cell Biol 82:163–173
Lang I, Schweizer A, Hiden U, Ghaffari-Tabrizi N, Hagendorfer G, Bilban M et al (2008) Human fetal placental endothelial cells have a mature arterial and a juvenile venous phenotype with adipogenic and osteogenic differentiation potential. Differentiation 76:1031–1043
Lang YD, Hung CL, Wu TY, Wang LF, Chen CM (2010) The renin-angiotensin system mediates hyperoxia-induced collagen production in human lung fibroblasts. Free Radic Biol Med 49:88–95
Lappas M, Mitton A, Permezel M (2010) In response to oxidative stress, the expression of inflammatory cytokines and antioxidant enzymes are impaired in placenta, but not adipose tissue, of women with gestational diabetes. J Endocrinol 204:75–84
Larsen M, Artym VV, Green JA, Yamada KM (2006) The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol 18:463–471
Legate KR, Wickstrom SA, Fassler R (2009) Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev 23:397–418
Lipfert L, Haimovich B, Schaller MD, Cobb BS, Parsons JT, Brugge JS (1992) Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp 125FAK in platelets. J Cell Biol 119:905–912
Loeffler I, Hopfer U, Koczan D, Wolf G (2011) Type VIII collagen modulates TGF-{beta}1-induced proliferation of mesangial cells. J Am Soc Nephrol 22:649–663
Loppnow H, Buerke M, Werdan K, Rose-John S (2011) Contribution of vascular cell-derived cytokines to innate and inflammatory pathways in atherogenesis. J Cell Mol Med 15:484–500
Madri JA, Pratt BM (1986) Endothelial cell-matrix interactions: in vitro models of angiogenesis. J Histochem Cytochem 34:85–91
Maubant S, Saint-Dizier D, Boutillon M, Perron-Sierra F, Casara PJ, Hickman JA et al (2006) Blockade of alpha v beta3 and alpha v beta5 integrins by RGD mimetics induces anoikis and not integrin-mediated death in human endothelial cells. Blood 108:3035–3044
Meredith JE Jr, Fazeli B, Schwartz MA (1993) The extracellular matrix as a cell survival factor. Mol Biol Cell 4:953–961
Nisato RE, Tille JC, Jonczyk A, Goodman SL, Pepper MS (2003) alphav beta 3 and alphav beta 5 integrin antagonists inhibit angiogenesis in vitro. Angiogenesis 6:105–119
Nodwell A, Carmichael L, Ross M, Richardson B (2005) Placental compared with umbilical cord blood to assess fetal blood gas and acid–base status. Obstet Gynecol 105:129–138
Parsons JT, Schaller MD, Hildebrand J, Leu TH, Richardson A, Otey C (1994) Focal adhesion kinase: structure and signalling. J Cell Sci Suppl 18:109–113
Pastor DM, Poritz LS, Olson TL, Kline CL, Harris LR, Koltun WA et al (2010) Primary cell lines: false representation or model system? A comparison of four human colorectal tumors and their coordinately established cell lines. Int J Clin Exp Med 3:69–83
Pichard V, Honore S, Kovacic H, Li C, Prevot C, Briand C et al (2001) Adhesion, actin cytoskeleton organisation and the spreading of colon adenocarcinoma cells induced by EGF are mediated by alpha2beta1 integrin low clustering through focal adhesion kinase. Histochem Cell Biol 116:337–348
Pietryga M, Biczysko W, Wender-Ozegowska E, Brazert J, Bieganska E, Biczysko R (2004) Ultrastructural examination of the placenta in pregnancy complicated by diabetes mellitus. Ginekol Pol 75:111–118
Priya S, Sudhakaran PR (2008) Cell survival, activation and apoptosis of hepatic stellate cells: modulation by extracellular matrix proteins. Hepatol Res 38:1221–1232
Read MA, Boura AL, Walters WA (1995) Effects of variation in oxygen tension on responses of the human fetoplacental vasculature to vasoactive agents in vitro. Placenta 16:667–678
Reiske HR, Kao SC, Cary LA, Guan JL, Lai JF, Chen HC (1999) Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase-promoted cell migration. J Biol Chem 274:12361–12366
Ren XD, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, Schwartz MA (2000) Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci 113(Pt 20):3673–3678
Rukosuev VS, Fokin EI, Milovanov AP (1989) Immunofluorescence study of the extracellular matrix of the human placenta. Ontogenez 20:171–178
Ruoslahti E (2002) Specialization of tumour vasculature. Nat Rev Cancer 2:83–90
Sanz L, Alvarez-Vallina L (2003) The extracellular matrix: a new turn-of-the-screw for anti-angiogenic strategies. Trends Mol Med 9:256–262
Sati L, Demir AY, Sarikcioglu L, Demir R (2008) Arrangement of collagen fibers in human placental stem villi. Acta Histochem 110:371–379
Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT (1994) Autophosphorylation of the focal adhesion kinase, pp 125FAK, directs SH2-dependent binding of pp 60src. Mol Cell Biol 14:1680–1688
Schiffrin EL (2001) Small artery remodeling in hypertension: can it be corrected? Am J Med Sci 322:7–11
Schwartz MA, Ginsberg MH (2002) Networks and crosstalk: integrin signalling spreads. Nat Cell Biol 4:E65–E68
Shenberger JS, Zhang L, Powell RJ, Barchowsky A (2007) Hyperoxia enhances VEGF release from A549 cells via post-transcriptional processes. Free Radic Biol Med 43:844–852
Simon MC, Keith B (2008) The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol 9:285–296
Skjot-Arkil H, Barascuk N, Register T, Karsdal MA (2010) Macrophage-mediated proteolytic remodeling of the extracellular matrix in atherosclerosis results in neoepitopes: a potential new class of biochemical markers. Assay Drug Dev Technol 8:542–552
Stromblad S, Cheresh DA (1996) Cell adhesion and angiogenesis. Trends Cell Biol 6:462–468
Van Rijen H, van Kempen MJ, Analbers LJ, Rook MB, van Ginneken AC, Gros D et al (1997) Gap junctions in human umbilical cord endothelial cells contain multiple connexins. Am J Physiol 272:C117–C130
Vartanian KB, Kirkpatrick SJ, Hanson SR, Hinds MT (2008) Endothelial cell cytoskeletal alignment independent of fluid shear stress on micropatterned surfaces. Biochem Biophys Res Commun 371:787–792
Vizza E, Goranova V, Heyn R, Correr S, Motta PM (2001) Extracellular fibrillar matrix architecture of human placental villi at term. Ital J Anat Embryol 106:317–323
Weidemann A, Johnson RS (2008) Biology of HIF-1alpha. Cell Death Differ 15:621–627
Weiss U, Cervar M, Puerstner P, Schmut O, Haas J, Mauschitz R et al (2001) Hyperglycaemia in vitro alters the proliferation and mitochondrial activity of the choriocarcinoma cell lines BeWo, JAR and JEG-3 as models for human first-trimester trophoblast. Diabetologia 44:209–219
Wickstrom SA, Veikkola T, Rehn M, Pihlajaniemi T, Alitalo K, Keski-Oja J (2001) Endostatin-induced modulation of plasminogen activation with concomitant loss of focal adhesions and actin stress fibers in cultured human endothelial cells. Cancer Res 61:6511–6516
Williamson JR, Tilton RG, Chang K, Kilo C (1988) Basement membrane abnormalities in diabetes mellitus: relationship to clinical microangiopathy. Diabetes Metab Rev 4:339–370
Yamada T, Isemura M, Yamaguchi Y, Munakata H, Hayashi N, Kyogoku M (1987) Immunohistochemical localization of fibronectin in the human placentas at their different stages of maturation. Histochemistry 86:579–584
Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF (2002) beta8 integrins are required for vascular morphogenesis in mouse embryos. Development 129:2891–2903
Acknowledgments
The authors are grateful to Heimo Strohmaier and Heike Knausz, from the Flow Cytometry Core Facility, and to Eleonore Fröhlich and Markus Absenger from the Microscopy Core Facility of the Center of Medical Research, Graz, Austria, for their expert technical assistance. LL received a PhD fellowship for Molecular Medicine from the Medical University Graz. UH and GD were supported by funds of the Oesterreichische Nationalbank (Anniversary Fund, project numbers: 13307 to UH, 10053, 12601 to GD). AH received funding from the Austrian Science Fund (FWF, Grant number P22521-B18).
Author information
Authors and Affiliations
Corresponding author
Additional information
G. Desoye and U. Hiden contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lassance, L., Miedl, H., Konya, V. et al. Differential response of arterial and venous endothelial cells to extracellular matrix is modulated by oxygen. Histochem Cell Biol 137, 641–655 (2012). https://doi.org/10.1007/s00418-012-0917-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-012-0917-4