Abstract
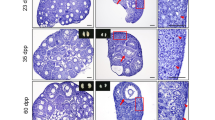
Folliculogenesis is a critical process during mammalian development, but little is known about the mechanisms underlying primordial follicular formation, growth, and activation. The objective of this study was to assess the effect of continuous insulin exposure on folliculogenesis in 16.5 dpc and 3 dpp mouse ovaries in vitro. The results demonstrated that 5 μg/ml insulin promotes the phosphorylation of Akt and the primordial follicular assembly and activation process via the InsR/PI3K/Akt signaling pathway in the 16.5 dpc fetal mouse ovaries cultured in vitro. However, the presence of 5 μg/ml insulin represses the phosphorylation of Akt and the follicular assembly and activation process in the 3 dpp mouse ovaries cultured in vitro. Furthermore, in vitro cultures of 3 dpp mouse ovaries with insulin can repress the expression of Pten. In conclusion, folliculogenesis was stage-specifically regulated by insulin via the Akt signaling pathway in vitro.




Similar content being viewed by others
References
Accili D, Arden KC (2004) FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117:421–426
Acevedo N, Ding J, Smith GD (2007) Insulin signaling in mouse oocytes. Biol Reprod 77:872–879
Arden KC, Biggs WH (2002) Regulation of the FoxO family of transcription factors by phosphatidylinositol-3 kinase-activated signaling. Arch Biochem Biophys 403:292–298
Blume-Jensen P, Hunter T (2001) Oncogenic kinase signaling. Nature 411:355–365
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857–868
Burgering BM, Medema RH (2003) Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol 73:689–701
Dong HS, Li L, Song ZH, Tang J, Xu B, Zhai XW, Sun LL, Zhang P, Li ZB, Pan QJ, Shi QH, Shen W (2009) Pre-meiotic fetal murine germ cells cultured in vitro form typical oocyte-like cells, but do not progress through meiosis. Theriogenology 72:219–231
Eppig JJ, O’Brien MJ (1996) Development in vitro of mouse oocytes from primordial follicles. Biol Reprod 54:197–207
SAS Institute (1996) SAS User’s Guide: Statistics, Version 7.0 ed., SAS Institute, Cary, NC
Kissel H, Timokhina I, Hardy MP, Rothschild G, Tajima Y, Soares V, Angeles M, Whitlow SR, Manova K, Besmer P (2000) Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J 19:1312–1326
Leslie NR, Downes CP (2004) PTEN function: how normal cells control it and tumour cells lose it. Biochem J 382:1–11
Liu K (2006a) Stem cell factor (SCF)-Kit mediated phosphatidylinositol 3 (PI3) kinase signaling during mammalian oocyte growth and early follicular development. Front Biosci 11:126–135
Liu K, Rajareddy S, Liu L, Jagarlamudi K, Boman K, Selstam G, Reddy P (2006b) Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: new roles for an old timer. Dev Biol 299:1–11
Liu L, Rajareddy S, Reddy P, Boman K, Shen Y, Lindgren P, Guo Y, Ottander U, Liu K (2007a) Phosphorylation and inactivation of glycogen synthase kinase-3 (GSK-3) by stem cell factor (SCF) in mouse oocytes during early follicular development. J Mol Endocrinol 38:137–146
Liu L, Rajareddy S, Reddy P, Jagarlamudi K, Shen Y, Gunnarson D, Boman K, Liu K (2007b) Infertility caused by follicular development retardation in mice with oocyte-specific expression of Foxo3a. Development 134:199–209
Maehama T, Dixon JE (1998) The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3, 4, 5-trisphosphate. J Biol Chem 273:13375–13378
Pepling ME, Spradling AC (2001) Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 234:339–351
Reddy P, Shen L, Ren C, Boman K, Lundin E, Ottander U, Lindgren P, Liu YX, Sun QY, Liu K (2005) Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev Biol 281:160–170
Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, Lan Z, Cooney AJ, Huhtaniemi I, Liu K (2008) Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 319:611–613
Shen W, Li L, Zhang DH, Ding MX, Deng HK (2006a) Mouse oocytes derived from fetal germ cells are competent to support the development of embryos by in vitro fertilization. Mol Reprod Dev 73:1312–1317
Shen W, Zhang DH, Qing TT, Cheng J, Bai DZ, Ding MX, Deng HK (2006b) Living offspring produced by mouse oocytes derived from premeiotic fetal germ cells. Biol Reprod 75:615–623
Shen W, Li L, Bai ZD, Pan QJ, Ding MX, Deng HK (2007) In vitro development of the mouse fetal germ cells to mature oocytes. Reproduction 134:223–231
Skinner MK (2005) Regulation of primordial follicle assembly and development. Hum Reprod Update 11:461–471
Song ZH, Min LJ, Pan QJ, Shi QH, Shen W (2009) Maternal imprinting during mouse oocyte growth in vivo and in vitro. Biochem Biophys Res Commun 387:800–805
Sun LL, Sun ZY, Zhang P, Zhai XW, Tang J, Pan QJ, Shi QH, Shen W (2010) Effect of insulin on oogenesis from mouse fetal germ cells in a serum-free 3D culture system. Reprod BioMed Online 20:11–25
Vlahos CJ, Matter WF, Hui KY, Brown RF (1994) A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1- benzopyran-4-one (LY294002). J Biol Chem 269:5241–5248
Zhang Q, Wang X, Wolgemuth DJ (1999) Developmentally regulated expression of cyclin D3 and its potential in vivo interacting proteins during murine gametogenesis. Endocrinology 140:2790–2800
Acknowledgments
We thank Dr. Paul Dyce for critical reading of the manuscript. Financial support was obtained from National Basic Research Program of China (973 Program, 2007CB947401 and 2006CB504003), National Nature Science Foundation (30700422), 863 Program of China (2008AA101003), Foundation of Taishan Scholar of Shandong Province.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
418_2010_708_MOESM1_ESM.tif
Supplement Fig. 1 Bisulfite sequencing analysis of the methylation status of Pten in the oocytes derived from 3 dpp mouse ovaries cultured in vitro with different concentrations of insulin for 5 days Supplementary material 1 (TIFF 171 kb)
Rights and permissions
About this article
Cite this article
Zhang, P., Chao, H., Sun, X. et al. Murine folliculogenesis in vitro is stage-specifically regulated by insulin via the Akt signaling pathway. Histochem Cell Biol 134, 75–82 (2010). https://doi.org/10.1007/s00418-010-0708-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-010-0708-8