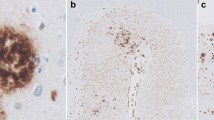
Abstract
Parenchymal accumulation of amyloid-beta (Aβ) is a hallmark pathological feature of Alzheimer’s disease. An emerging hypothesis is that blood-to-brain delivery of Aβ may increase with compromised blood–brain barrier integrity. In plasma, substantial Aβ is associated with triglyceride-rich lipoproteins (TRLs) secreted by the liver and intestine. Utilizing apolipoprotein B as an exclusive marker of hepatic and intestinal TRLs, here we show utilizing an highly sensitive 3-dimensional immuno-microscopy imaging technique, that in APP/PS1 amyloid transgenic mice, concomitant with substantially increased plasma Aβ, there is a significant colocalization of apolipoprotein B with cerebral amyloid plaque. The findings are consistent with the possibility that circulating lipoprotein-Aβ contributes to cerebral amyloidosis.


Similar content being viewed by others
References
Bolte S, Cordelieres FP (2006) A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224:213–232
Burgess BL, McIsaac SA, Naus KE, Chan JY, Tansley GH, Yang J, Miao F, Ross CJ, van Eck M, Hayden MR, van Nostrand W, St George-Hyslop P, Westaway D, Wellington CL (2006) Elevated plasma triglyceride levels precede amyloid deposition in Alzheimer’s disease mouse models with abundant A beta in plasma. Neurobiol Dis 24:114–127
Comeau JW, Costantino S, Wiseman PW (2006) A guide to accurate fluorescence microscopy colocalization measurements. Biophys J 91:4611–4622
Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S (2004) Automatic and quantitative measurement of protein–protein colocalization in live cells. Biophys J 86:3993–4003
Crossgrove JS, Li GZ, Zheng W (2005) The choroid plexus removes beta-amyloid from brain cerebrospinal fluid. Exp Biol Med 230:771–776
Cullen KM (1997) Perivascular astrocytes within Alzheimer’s disease plaques. Neuroreport 8:1961–1966
Deane R, Sagare A, Hamm K, Parisi M, LaRue B, Guo H, Wu Z, Holtzman DM, Zlokovic BV (2005) IgG-assisted age-dependent clearance of Alzheimer’s amyloid beta peptide by the blood–brain barrier néonatal Fc receptor. J Neurosci 25:11495–11503
Ellis RJ, Olichney JM, Thal LJ, Mirra SS, Morris JC, Beekly D, Heyman A (1996) Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: the CERAD experience, part XV. Neurology 46:1592–1596
Galloway S, Jian L, Johnsen R, Chew S, Mamo JC (2007) Beta-amyloid or its precursor protein is found in epithelial cells of the small intestine and is stimulated by high-fat feeding. J Nutr Biochem 4:279–284
Kalmijn SJ (2000) Fatty acid intake and the risk of dementia and cognitive decline: a review of clinical and epidemiological studies. J Nutr Health Aging 4:202–207
Koudinov AR, Koudinova NV (1997) Alzheimer’s soluble amyloid beta protein is secreted by HepG2 cells as an apolipoprotein. Cell Biol Int 21:265–271
LaRue B, Hogg E, Sagare A, Jovanovic S, Maness L, Maurer C, Deane R, Zlokovic BV (2004) Method for measurement of the blood–brain barrier permeability in the perfused mouse brain: application to amyloid-beta peptide in wild type and Alzheimer’s Tg2576 mice. J Neurosci Methods 138:233–242
Levin-Allerhand JA, Lominska CE, Smith JD (2002) Increased amyloid levels in APPSWE transgenic mice treated chronically with a physiological high-fat high-cholesterol diet. J Nutr Health Aging 6:315–319
Mackic JB, Bading J, Ghiso J, Walker L, Wisniewski T, Frangione B, Zlokovic BV (2002) Circulating amyloid-beta peptide crosses the blood–brain barrier in aged monkeys and contributes to Alzheimer’s disease lesions. Vascul Pharmacol 38:308–313
Mamo JC, Jian L, James AP, Flicker L, Esselmann H, Wiltfang J (2008) Plasma lipoprotein beta-amyloid in subjects with Alzheimer’s disease or mild cognitive impairment. Ann Clin Biochem 45:395–403
Manders EM, Stap J, Brakenhoff GJ, van Driel R, Aten JA (1992) Dynamics of three-dimensional replication patterns during the S-phase, analysed by double labelling of DNA and confocal microscopy. J Cell Sci 103:857–862
Manders EMM, Verbekk FJ, Aten JA (1993) Measurement of co-localization of objects in dual-colour confocal images. J Microsc 169:375–382
Namba Y, Tsuchiya H, Ikeda K (1992) Apolipoprotein B immunoreactivity in senile plaque and vascular amyloids and neurofibrillary tangles in the brains of patients with Alzheimer’s disease. Neurosci Lett 134:264–266
Sparks DL, Scheff SW, Hunsaker JC 3rd, Liu H, Landers T, Gross DR (1994) Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol 126:88–94
Su GC, Arendash GW, Kalaria RN, Bjugstad KB, Mullan M (1999) Intravascular infusions of soluble beta-amyloid compromise the blood–brain barrier, activate CNS glial cells and induce peripheral hemorrhage. Brain Res 818:105–117
Takechi R, Galloway S, Pallebage-Gamarallage MMS, Mamo JCL (2008a) Chylomicron amyloid-beta in the aetiology of Alzheimer’s disease. Atheroscler Suppl 9:19–25
Takechi R, Galloway S, Pallebage-Gamarallage MM, Johnsen RD, Mamo JC (2008b) Three-dimensional immunofluorescent double labelling using polyclonal antibodies derived from the same species: enterocytic colocalization of chylomicrons with Golgi apparatus. Histochem Cell Biol 129:779–784
Thomas T, McLendon C, Sutton ET, Thomas G (1997) Cerebrovascular endothelial dysfunction mediated by beta-amyloid. Neuroreport 8:1387–1391
Wisniewski HM, Vorbrodt AW, Wegiel J (1997) Amyloid angiopathy and blood–brain barrier changes in Alzheimer’s disease. Ann NY Acad Sci 826:161–172
Acknowledgments
This research was financially supported by the Australian Technology Network Centre for Metabolic Fitness (Curtin University node).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takechi, R., Galloway, S., Pallebage-Gamarallage, M. et al. Three-dimensional colocalization analysis of plasma-derived apolipoprotein B with amyloid plaques in APP/PS1 transgenic mice. Histochem Cell Biol 131, 661–666 (2009). https://doi.org/10.1007/s00418-009-0567-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-009-0567-3