Abstract
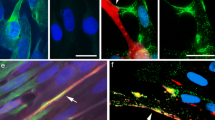
Previously we have shown that during in vivo muscle regeneration differentiating rat primary myoblasts transiently upregulate connexin43 (Cx43) gap junctions and leave cell cycle synchronously. Here, we studied the temporal regulation of Cx expression in relation to functional dye coupling in allogenic primary myoblast cultures using western blotting, immuno-confocal microscopy and dye transfer assays. As in vivo, Cx43 was the only Cx isotype out of Cx26, 32, 37, 40, 43 and 45 found in cultured rat myoblasts by immunostaining. Cultured myoblasts showed similar temporal regulation of Cx43 expression and phenotypic maturation to those regenerating in vivo. Cx43 protein was progressively upregulated in prefusion myoblasts, first by the cytoplasmic assembly in sparse myoblast meshworks and then in cell membrane particles in aligned cells. Dye injection using either Lucifer Yellow alone, Cascade Blue with a non-junction permeant FITC-dextran revealed an extensive gap junction coupling between the sparse interacting myoblasts and a reduced communication between the aligned, but still prefused cells. The aligned myoblasts, uniformly upregulate p21waf1/cip1 and p27kip1 cell cycle control proteins. Taken together, in prefusion myoblasts less membrane-bound Cx43 was found to mediate substantially more efficient dye coupling in the growing cell fraction than those in the aligned post-mitotic myoblasts. These and our in vivo results in early muscle differentiation are consistent with the role of Cx43 gap junctions in synchronizing cell cycle control of myoblasts to make them competent for a coordinated syncytial fusion.





Similar content being viewed by others
References
Albright CD, Kuo J, Jeong S (2001) cAMP enhances Cx43 gap junction formation and function and reverses choline deficiency apoptosis. Exp Mol Pathol 71:34–39
Araya R, Eckhardt D, Maxiener S, Kruger O, Theiss M, Willecke K, Saez JC (2005) Expression of connexins during differentiation and regeneration of skeletal muscle: functional relevance of connexin43. J Cell Sci 118:27–37
Armstrong DL, Turin L, Warner AE (1983) Muscle activity and the loss of electrical coupling between striated muscle cells in Xenopus embryos. J Neurosci 3:1414–1421
Azzam EI, de Toledo SM, Little JB (2001) Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to non-irradiated cells. Proc Natl Acad Sci USA 98:473–478
Baek HJ, Jeon YJ, Kim HS, Kang MS, Chung CH, Ha DB (1994) Cyclic AMP negatively modulates both Ca2+/calmodulin-dependent phosphorylation of the 100-kDa protein and membrane fusion of chick embryonic myoblasts. Dev Biol 165:178–184
Balogh S, Naus CC, Merrifield PA (1993) Expression of gap junctions in cultured rat L6 cells during myogenesis. Dev Biol 155:351–360
Becker DL, Evans WH, Green CR, Warner A (1995) Functional analysis of amino acid sequences in connexin43 involved in intercellular communication through gap junctions. J Cell Sci 108:1455–1467
Becker DL, McGonnell I, Makarenkova HP, Patel K, Tickle C, Lorimer J, Green CR (1999) Roles for alpha 1 connexin in morphogenesis of chick embryos revealed using a novel antisense approach. Dev Genet 24:33–42
Berthoud VM, Singh R, Minogue PJ, Ragsdale CW, Beyer EC (2004) Highly restricted pattern of connexin36 expression in chick somite development. Anat Embryol (Berl) 209:11–18
Beyer EC (1990) Molecular cloning and developmental expression of two chick embryo gap junction proteins. J Biol Chem 265:14439–14443
Bischoff R (1990) Cell cycle commitment of rat muscle satellite cells. J Cell Biol 111:201–207
Boitano S, Dirksen ER, Evans WH (1998) Sequence-specific antibodies to connexins block intercellular calcium signaling through gap junctions. Cell Calcium 23:1–9
Clairmount A, Sies H (1997) Evidence for posttranscriptional effect of retinoic acid on connexin43 gene expression via the 3′-untranslated region. FEBS Lett 419:268–270
Constantin B, Cronier L (2000) Involvement of gap junctional communication in myogenesis. Int Rev Cytol 196:1–65
Constantin B, Cronier L, Raymond G (1997) Transient involvement of gap junctional communication before fusion of newborn rat myoblasts. C R Acad Sci III 320:35–40
Coppen S, Dupont E, Rothery S, Severs N (1998) Connexin45 expression is preferentially associated with the ventricular conduction system in mouse and rat heart. Circ Res 82:232–243
Dahl E, Winterhager E, Traub O, Willecke K (1995) Expression of gap junction genes, connexin40 and connexin43, during fetal mouse development. Anat Embryol (Berl) 191:267–278
David JD, See WM, Higginbotham CA (1981) Fusion of chick embryo skeletal myoblasts: role of calcium influx preceding membrane union. Dev Biol 82:297–307
David JD, Faser CR, Perrot GP (1990) Role of protein kinase C in chick embryo skeletal myoblast fusion. Dev Biol 139:89–99
Darrow BJ, Fast VG, Kleber AG, Beyer EC, Saffitz JE (1996) Functional and structural assessment of intercellular communication. Increased conduction velocity and enhanced connexin expression in dibutyryl cAMP-treated cultured cardiac myocytes. Circ Res 79:174–183
Farnazeh F, Entwistle A, Zalin RJ (1989) Protein kinase C mediates the hormonally regulated plasma membrane fusion of avian embryonic skeletal muscle. Exp Cell Res 181:298–304
Gorbe A, Dux L, Krenacs T (2000) Direct cell–cell communication through gap junctions in the regenerating skeletal muscle. J Physiol (Lond) 256(Suppl S):22–23
Gorbe A, Becker DL, Dux L, Stelkovics E, Krenacs L, Bagdi L, Krenacs T (2005) Transient upregulation of connexin43 gap junctions and synchronized cell cycle control precede myoblast fusion in regenerating skeletal muscle in vivo. Histochem Cell Biol 123:573–583
Goodenough DA, Paul DL (2003) Beyond the gap: functions of unpaired connexin channels. Nat Rev Mol Cell Biol 4:285–294
Grounds MD (1999) Muscle regeneration: molecular aspects and therapeutic implications. Curr Opin Neurol 12:535–543
Hawke TJ, Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91:534–551
Kalderon N, Epstein ML, Gilula NB (1977) Cell-to-cell communication and myogenesis. J Cell Biol 75:788–806
Keresztes M, Haggblad J, Heilbronn E (1991) Basal and ATP-stimulated phosphoinositol metabolism in fusing rat skeletal muscle cells in culture. Exp Cell Res 196:362–364
Kitzmann M, Fernandez A (2001) Crosstalk between cell cycle regulators and the myogenic factor MyoD in skeletal myoblasts. Cell Mol Life Sci 58:571–579
Koffler L, Roshong S, Park KI, Cesen-Cummings K, Thompson DC, Dwyer-Nield LD, Rice P, Mamay C, Malkinson AM, Ruch RJ (2000) Growth inhibition in G1 and altered expression of cyclin D1 and p27 kip1 after forced connexin expression in lung and liver carcinoma cells. J Cell Biochem 79:347–354
Krenacs T, van Dartel M, Lindhout E, Rosendaal M (1997) Direct cell/cell communication in the lymphoid germinal center: connexin43 gap junctions functionally couple follicular dendritic cells to each other and to B lymphocytes. Eur J Immunol 27:1489–1497
Kumar NM, Gilula NB (1996) The gap junction communication channel. Cell 84:381–388
Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG (2000) Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol 149:1503–1512
Lefaucheur JP, Sebille A (1995) The cellular events of injured muscle regeneration depend on the nature of the injury. Neuromuscul Disord 5:501–509
Lowry OH, Rosebrough HJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
von Maltzahn J, Euwens C, Willecke K, Sohl G (2004) The novel mouse connexin 39 gene is expressed in developing striated muscle fibers. J Cell Sci 117:5381–5392
Marchal S, Cassar-Malek I, Magaud JP, Rouault JP, Wrutniak C, Cabello G (1995) Stimulation of avian myoblast differentiation by triiodothyronine: possible involvement of the cAMP pathway. Exp Cell Res 220:1–10
Mege RM, Goudou D, Giaume C, Nicolet M, Rieger F (1994) Is intercellular communication via gap junctions required for myoblast fusion? Cell Adhes Commun 2:329–343
Milks LC, Kumar NM, Houghten R, Unwin N, Gilula NB (1988) Topology of the 32-kd liver gap junction protein determined by site-directed antibody localization. EMBO J 7:2967–2975
Miner JH, Wold B (1990) Herculin, a fourth member of the MyoD family of myogenic regulatory factors. Proc Natl Acad Sci USA 87:1089–1093
Ostrovsky O, Bengal E (2003) The mitogen-activated protein kinase cascade promotes myoblast cell survival by stabilizing the cyclin-dependent kinase inhibitor, p21WAF1 protein. J Biol Chem 278:21221–21231
Proulx A, Merrifield PA, Naus CC (1997) Blocking gap junctional intercellular communication in myoblasts inhibits myogenin and MRF4 expression. Dev Genet 20:133–144
Rando TA, Blau HM (1994) Primary mouse myoblast purification, characterization and transplantation for cell-mediated gene therapy. J Cell Biol 125:1275–1287
Rash JE, Fambrough D (1973) Ultrastructural and electrophysiological correlates of cell coupling and cytoplasmic fusion during myogenesis in vitro. Dev Biol 30:166–186
Rhodes SJ, Konieczky SF (1989) Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev 3:2050–2061
Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R (1993) MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 75:1351–1359
Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer E (2003) Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 83:1359–1400
Schmalbruch H (1982) Skeletal muscle fibers of newborn rats are coupled by gap junctions. Dev Biol 91:485–490
Shin YJ, Woo JH, Chung CH, Kim HS (2000) Retinoic acid and its geometrical isomers block both growth and fusion of L6 myoblasts by modulating the expression of protein kinase A. Mol Cells 10:162–168
Sohl G, Willecke K (2004) Gap junctions and the connexin protein family. Cardiovasc Res 62:228–232
Stout C, Goodenough DA, Paul DL (2004) Connexins: functions without junctions. Cur Opin Cell Biol 16:507–512
Yeh HI, Dupont E, Coppen S, Rothery S, Severs N (1997) Gap junction localization and connexin expression in cytochemically identified endothelial cells of arterial tissue. J Histochem Cytochem 45:539–550
Yeh HI, Rothery S, Dupont E, Coppen S, Severs N (1998) Individual gap junction plaques contain multiple connexins in arterial endothelium. Circ Res 83:1248–1263
Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G (2002) Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem 383:725–737
Wright WE, Sassoon DA, Lin VK (1989) Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell 56:607–617
Wright CS, Becker DL, Lin JS, Warner AE, Hardy K (2001) Stage-specific and differential expression of gap junctions in the mouse ovary: connexin-specific roles of follicular regulation. Reproduction 121:77–88
Zalin RJ, Montague W (1974) Changes in adenylate cyclase, cyclic AMP, and protein kinase levels in chick myoblasts, and their relationship to differentiation. Cell 2:103–108
Zhang J-T, Nicholson BJ (1989) Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J Cell Biol 109:3391–3401
Zhang YW, Morita I, Ikeda M, Ma KW, Murota S (2001) Connexin43 suppresses proliferation of osteosarcoma U2OS cells through post-transcriptional regulation of p27. Oncogene 20:4138–4149
Acknowledgements
The authors are grateful to professors Howard Evans (University of Wales, Cardiff) and Nicholas Severs (National Heat and Lung Institute, London) Robert Gourdie (MUSC) for sharing their connexin specific antibodies. Mr. Daniel Ciantar (UCL), Mrs. Elizabet Balazshazi (Department of Biochemistry, Szeged), Aniko Sarro, and Katalin Danyi (Bay Zoltán Foundation, Szeged) for their skilful technical assistance. This study was supported by grants from The Royal Society in the UK (15109), and by OTKA T32928, ETT 42102/2003 and RET 08/2004 in Hungary.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gorbe, A., Becker, D.L., Dux, L. et al. In differentiating prefusion myoblasts connexin43 gap junction coupling is upregulated before myoblast alignment then reduced in post-mitotic cells. Histochem Cell Biol 125, 705–716 (2006). https://doi.org/10.1007/s00418-005-0121-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-005-0121-x