Abstract
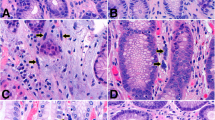
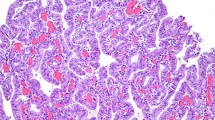
The present study was undertaken to clarify the histochemical and ultrastructural properties and the three-dimensional distribution of the smooth muscle cells (SMCs) located in the lamina propria (LP) of the human gastric mucosa. Standard paraffin sections obtained from stomachs surgically resected for gastric cancer were immunostained for alpha-smooth muscle actin (alpha-SMA), vimentin, desmin, laminin, and type IV collagen. In addition, 100-μm-thick sections were fluorostained for alpha-SMA and CD34, while three-dimensional images were prepared by confocal laser scanning microscope. Ultrastructural studies were carried out using normal gastric biopsy specimens. The results indicated that SMCs in the LP differed between the upper and lower regions, SMCs in the lower LP being fairly typical SMCs, whereas those in the upper LP had apparently lost reactivity for desmin but gained that for vimentin. The basal lamina became sparser, but a fibronexus was occasionally seen in SMCs in the upper LP. Three-dimensional images revealed bundles of SMCs in the upper LP encircling several foveolae to form acinus-like structures and, in the upper LP, SMCs branching into fine fibrils with a brush-like (corpus) or besom-like (i.e., a twiggy “witch’s broom”) appearance (antrum).








Similar content being viewed by others
References
Eyden BP (1993) Brief review of the fibronexus and its significance for myofibroblastic differentiation and tumor diagnosis. Ultrastruct Pathol 17:611–622
Eyden B (2001) The fibronexus in reactive and tumoral myofibroblasts: further characterization by electron microscopy. Histol Histopathol 16:57–70
Fletcher CDM (1999) Myofibroblastic sarcoma. Author’s reply. Am J Surg Pathol 23:1143–1435
Hayama M, Ota H, Toki T, Ishii K, Honda T, Momose M, Nakata R (2002) Cell kinetic study of the endometrium by nonisotopic in situ hybridization for histone H3 messenger RNA and immunohistochemistry for Ki-67 and for estrogen and progesterone receptors. Anat Rec 266:234–240
Hidaka E, Ota H, Katsuyama T, Nakayama J (2000) Coexistence of gland mucous cell-type mucin and lysozyme in gastric gland mucous cells. Histochem Cell Biol 113:91–98
Li A, Hasui K, Yonezawa S, Tanaka S, Sato E (1999) Immunohistochemical analysis of pericryptal fibroblast sheath and proliferating epithelial cells in human colorectal adenomas and carcinomas with adenoma components. Pathol Int 49:426–434
Maeyama H, Furuwatari C, Ota H, Akamatsu T, Nakayama J, Katsuyama T (1997) Histone H3 messenger RNA in situ hybridization for identifying proliferating cells in formalin-fixed rat gastric mucosa. Histochem J 29:867–873
Merchenthaler I, Stankovics J, Gallyas F (1989) A highly sensitive one-step method for silver intensification of the nickel-diaminobenzidine end-product of peroxidase reaction. J Histochem Cytochem 37:1563–1565
Schurch W, Seemayer T, Gabbiani G (1998) The myofibroblast: a quarter century after its discovery. Am J Surg Pathol 22:141–147
Shimizu Y, Yamamichi N, Saito K, Watanabe A, Ito T, Yamamichi-Nishina M, Mizutani M, Yahagi N, Suzuki T, Sasakawa C, Yasugi S, Ichinose M, Iba H (2003) Kinetics of c-src-induced epithelial-mesenchymal transition in developing glandular stomach. Oncogene 22:884–893
Synnerstad I, Ekblad E, Sundler F, Holm L (1998) Gastric mucosal smooth muscles may explain oscillations in glandular pressure: role of vasoactive intestinal peptide. Gastroenterology 114:284–294
Tsuchihashi Y, Tani T, Maruyama K, Yorioka S, Okada K, Sudo H, Ashihara T, Fujita S, Kawai K (1988) Structural alternations of mucosal microvascular system in human chronic gastritis. In: Manabe H, Zweifach BW, Messemer K (eds) Microcirculation in circulatory disorders. Springer, Berlin Heidelberg New York, pp 161–169
Tsuzuki S, Ota H, Hayama M, Sugiyama A, Akamatsu T, Kawasaki S (2001) Proliferation of alpha-smooth muscle actin-containing stromal cells (myofibroblasts) in the lamina propria subjacent to intraepithelial carcinoma of the esophagus. Scand J Gastroenterol 36:86–91
Watanabe S, Hirose M, Wang X, Kobayashi O, Nagahara A, Murai T, Iwazaki R, Miwa H, Miyazaki A, Sato N (2000) Epithelial-mesenchymal interaction in gastric mucosal restoration. Gastroenterology 35(suppl XII):65–68
Acknowledgements
The authors thank Dr. Takayuki Honda (Department of Laboratory Medicine, Shinshu University School of Medicine, Shinshu University, Matsumoto, Japan) for extremely helpful discussion and comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arai, K., Ota, H., Hidaka, E. et al. Histochemical, ultrastructural, and three-dimensional observation of smooth muscle cells in human gastric mucosa. Histochem Cell Biol 121, 229–237 (2004). https://doi.org/10.1007/s00418-004-0628-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-004-0628-6