Abstract
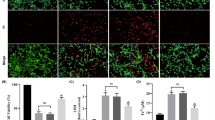
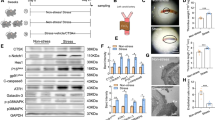
The present investigation provides for the first time, unambiguous information on the occurrence of hypoxia-inducible factors (HIF-1α and HIF-1β proteins) in normoxia (Nx) and their interaction with hypoxia (Hx) and intracellular Fe2+ chelation in the rat carotid body (CB) glomus cells. HIF-1α bound to HIF-1β translocated into the nucleus is identified on the basis of immunohistochemistry and immunofluorescence. In Nx, a weak expression of HIF-1α was observed in CB glomus cells. However, exposure of CB and glomus cells to Hx (Po2≃7 Torr) and Nx with ciclopirox olamine (CPX, 5 μM) for 1 h showed a significant (P<0.001) increase in HIF-1α protein. The CBs and glomus cells exposed to Nx, Hx, and Nx with CPX showed a constant level of HIF-1β protein expression. HIF-1α subunit is continuously synthesized and degraded under normoxic conditions, while it accumulates rapidly following exposure to low oxygen tensions. Hydroxylation of HIF-1α by prolyl hydroxylase for proteasomal degradation was dependent on iron, 2-oxoglutarate, and oxygen concentration. The intracellular iron that acts as a cofactor for prolyl hydroxylase activity belongs to the labile iron pool and can be easily chelated. Thus, chelation of intracellular labile iron by CPX in Nx significantly increased HIF-1α in CB glomus cells. Thus, the results are consistent with the hypothesis that HIF-1α which is present in the glomus cells translocates to the nucleus during exposure to Hx and to CPX in Nx.





Similar content being viewed by others
References
Acker H, Berchner-Pfannschmidt U, Wotzlaw C, Huckstorf C, Streller T (2003) Optical analysis of the oxygen-sensing pathway. In: Lahiri S, Semenza GL, Prabhakar NR (eds) Oxygen-sensing: responses and adaptation to hypoxia, vol 175. Lung biology in health and disease. Marcel-Dekker, New York, pp 507−517
Baby SM, Roy A, Mokashi AM, Lahiri S (2003) Effect of iron chelation on hypoxia induction factors (HIF-1α and HIF-1β) in adult rat carotid body (CB) glomus cells. FASEB J 17:A16
Bergeron M, Evans SM, Sharp FR, Koch CJ, Lord EM, Ferriero DM (1999) Detection of hypoxic cells with the 2-nitroimidazole, EF5, correlates with early redox changes in rat brain after perinatal hypoxia-ischemia. Neuroscience 89:1357–1366
Bruick RK, McKnight SL (2001) A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294:1337–1340
Chilov D, Camenisch G, Kvietikova I, Ziegler U, Gassmann M, Wenger RH (1999) Induction and nuclear translocation of hypoxia-inducible factor-1 (HIF-1): heterodimerization with ARNT is not necessary for nuclear accumulation of HIF-1α. J Cell Sci 112:1203–1212
Crews ST (1998) Control of cell lineage-specific development and transcription by bHLH-PAS proteins. Genes Dev 12:607–620
Daudu PA, Roy A, Rozanov C, Mokashi A, Lahiri S (2002) Extra- and intracellular free iron and the carotid body responses. Respir Physiol Neurobiol 130:21–31
Drutel G, Heron A, Kathmann M, Gros C, Mace S, Plotkine M, Schwartz JC, Arrang JM (1999) ARNT2, a transcription factor for brain neuron survival? Eur J Neurosci 11:1545–1553
Drutel G, Kathmann M, Heron A, Gros C, Mace S, Schwartz JC, Arrang JM (2000) Two splice variants of the hypoxia-inducible factor HIF-1α as potential dimerization partners of ARNT2 in neurons. Eur J Neurosci 12:3701–3708
Harris AL (2002) Hypoxia-A key regulatory factor in tumor growth. Nat Rev 2:38–47
Huang LE, Gu J, Schau M, Bunn HF (1998) Regulation of hypoxia-inducible factor-1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A 95:7987–7992
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kalelin WG Jr (2001) HIF-1α targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292:464–468
Jaakkola P, Mole DR, Tian YM, Wilson MI, Giebert J, Gaskell SJ, Kriegsheim AV, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ (2001) Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468–472
Jewell UR, Kvietikova I, Scheid A, Bauer C, Wenger RH, Gassmann M (2001) Induction of HIF-1α in response to hypoxia is instantaneous. FASEB J 15:1312–1314
Kakinuma Y, Miyauchi T, Yuki K, Murakoshi N, Goto K, Yamaguchi I (2001) Novel molecular mechanism of increased myocardial endothelin-1 expression in the failing heart involving the transcriptional factor hypoxia-inducible factor-1α induced for impaired myocardial energy metabolism. Circulation 103:2387–2394
Kallio PJ, Pongratz I, Gradin K, McGuire J, Poellinger L (1997) Activation of hypoxia-inducible factor-1α: posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc Natl Acad Sci U S A 94:5667–5672
Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway RC, Conway JW (2000) Activation of HIF-1α ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc Natl Acad Sci U S A 97:10430–10435
Lahiri S, Rumsey WL, Wilson DF, Iturriaga R (1993) Contribution of in vivo microvascular Po2 in the cat carotid body. J Appl Physiol 75:1035–1043
Lahiri S, Rozanov C, Roy A, Storey B, Buerk DG (2001) Regulation of oxygen sensing in peripheral arterial chemoreceptors. Int J Biochem Cell Biol 33:755–774
Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML (2002) Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 295:858–861
Li J, Roy A, Daudu PA, Lahiri S (2002) Ciclopirox olamine-induced inhibition of K+ current in PC-12 cells. FASEB J 15:A817
Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC (1997) Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 386:403–407
Mokashi A, Roy A, Rozanov C, Osanai S, Storey BT, Lahiri S (1998) High PCO does not alter pH i , but raises [Ca2+] i in cultured rat carotid body glomus cells in the absence and presence of CdC12. Brain Res 803:194–197
Norris ML, Millhorn DE (1995) Hypoxia-induced protein binding to O2-responsive sequences on the tyrosine hydroxylase gene. J Biol Chem 270:23774–23779
Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kalein WG (2000) Ubiquitination of hypoxia-inducible factor requires direct binding to the β-domain of the von Hippel-Lindau protein. Nat Cell Biol 2:423–427
Prabhakar NR (2000) Oxygen sensing by the carotid body chemoreceptors. J Appl Physiol 88:2287–2295
Ren X, Dorrington KL, Maxwell PH, Robbins PA (2000) Effects of desferrioxamine on serum erythropoietin and ventilatory sensitivity to hypoxia in humans. J Appl Physiol 89:680–686
Roy A, Li J, Al-Mehdi A, Mokashi A, Lahiri S (2002) Effect of hypoxia on glomus cell Em and ψm as measured by fluorescence imaging. J Appl Physiol 93:1987–1998
Rumsey WL, Iturriaga R, Spergel D, Lahiri S, Wilson DF (1991) Optical measurements of the dependence of chemoreception on oxygen pressure in the cat carotid body. Am J Physiol 261:C614–C622
Salceda S, Caro J (1997) Hypoxia-inducible factor 1α (HIF-1α) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem 272:22642–22647
Semenza GL (2001) Hypoxia-inducible factor-1: oxygen homeostasis and disease pathophysiology. Trends Mol Med 7:345–353
Srinivas V, Leshchinsky I, Sang N, King MP, Minchenko A, Caro J (2001) Oxygen sensing and HIF-1 activation does not require an active mitochondrial respiratory chain electron transfer pathway. J Biol Chem 276:21995–21998
Stroka DM, Burkhardt T, Desbailets I, Wenger RH, Neil DA, Bauer C, Gassmann M, Candinas D (2001) HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J 15:2445–2453
Sutter CH, Laughner E, Smenza GL 2000) Hypoxia-inducible factor-α protein expression is controlled by oxygen-regulated ubiquitination that is disrupted by deletions and missense mutations. Proc Natl Acad Sci U S A 97:748–753
Wang GL, Semenza GL (1993) Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood 82:3610–3615
Wanner RM, Spielmann P, Stroka DM, Camenisch G, Camenisch I, Scheid A, Houck DR, Bauer C, Gassmann M, Wenger RH (2000) Epolones induce erythropoietin expression via hypoxia-inducible factor-1α activation. Blood 96:1558–1565
Wilson DF, Evans SM, Rozanov C, Roy A, Koch CJ, Laughlin KM, Lahiri S (2000) Intracellular Po2 of the carotid body. In: Lahiri S, Prabhakar NR, Foster RE (eds) Oxygen sensing molecule to man, vol 475. Kluwer Academic/Plenum, New York, pp 637–644
Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA (2001) Molecular regulation of the endothelin-1 gene by hypoxia: contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, and p300/CBP. J Biol Chem 276:12645–12653
Zhu H, Bunn HF (2001) Signal transduction. How do cells sense oxygen? Science 292:449–451
Acknowledgements
The authors are thankful to Dr. L. Kubin, Department of Animal Biology, for his excellent guidance in designing the experimental protocol, and to Mrs. Kathy Notarfrancesco, Institute for Environmental Medicine, Juli Burns, Department of Pathology, and Maggie Grimes, Department of Physiology, University of Pennsylvania for their skillful technical assistance. S.M.B. is a recipient of an NRSA fellowship award. This work was supported by NIH training grants T-32-HL07027 and in part by ONR N-00014-01-0948, R01-HL50180, and R37-HL43413.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baby, S.M., Roy, A., Mokashi, A.M. et al. Effects of hypoxia and intracellular iron chelation on hypoxia-inducible factor-1α and -1β in the rat carotid body and glomus cells. Histochem Cell Biol 120, 343–352 (2003). https://doi.org/10.1007/s00418-003-0588-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-003-0588-2