Abstract
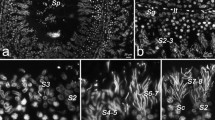
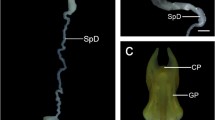
The expression of cathepsin H (CH) in differentiating rat spermatids was studied by an immunoelectron microscopic technique. Cathepsin H was detected in the acrosome throughout differentiation steps but cathepsins B, D, and L and lysosomal membrane protein (LGP107) were not. Early in the formation of the acrosome, CH signals were observed in Golgi vesicles but not in acrosomal vesicles. At steps 3–4, CH signals were associated with a fibrous material attached to the inner surface of the vesicle membrane on the Golgi side. At steps 5–6, this fibrous material accumulated to form an electron-dense sheet to which CH signals were confined. The rest of the acrosome was negative for the enzyme. At steps 11–12, the CH-positive fibrous sheet expanded from the apical to the ventral side of the sperm head. After step 16, the surface of outer dense fibers in the flagellar axoneme and reticulated bodies were stained for CH. In epididymal sperm, CH signals were detected in the acrosome as well as on the surface of the outer dense fibers running from the middle to the principal piece. By immunofluorescence staining, CH was found to be localized to the acrosome, middle piece, and principal piece.





Similar content being viewed by others
References
Allison AC, Hartree EF (1970) Lysosomal enzymes in the acrosome and their possible role in fertilization. J Reprod Fertil 21:501–515
Bowen RH (1922) On the idiosome, Golgi apparatus and acrosome in the male germ cells. Anat Rec 24:158–180
Brökelmann J (1963) Fine structure of germ cells and Sertoli cells during the cycle of the seminiferous epithelium in the rat. Z Zellforsch 59:820–850
Calvin HI (1976) Isolation and subfractionation of mammalian sperm heads and tails. Methods Cell Biol 13:85–104
Chung SSW, Zhu L-J, Mo M-Y, Silvestrini B, Lee WM, Cheng CY (1998) Evidence for cross-talk between Sertoli and germ cells using selected cathepsins as markers. J Androl 19:686–703
Clermont Y (1972) Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev 52:198–236
Clermont Y, Lalli M, Rambourg A (1981) Ultrastructural localization of nicotinamide adenine dinucleotide phosphatase (NADPase), thiamine pyrophosphatase (TTPase), and cytidine monophosphatase (CMPase) in the Golgi apparatus of early spermatids of the rat. Anat Rec 201:613–622
Clermont Y, Oko R, Hermo L (1990) Immunocytochemical localization of proteins utilized in the formation of outer dense fibers and fibrous sheath in rat spermatids: an electron microscope study. Anat Rec 227:447–457
de Kretser DM, Kerr JB (1988) The cytology of the testis. In: Knobil E, Neill J (eds) The physiology of reproduction. Raven, New York, pp 837–932
Erickson R, Martin SR (1974) The relationship to mouse spermatozoal to mouse testicular cathepsins. Arch Biochem Biophys 165:114–120
Gardner PJ (1966) Fine structure of the seminiferous tubule of the Swiss mouse. The spermatid. Anat Rec 155:235–250
Gatenby JB, Beams HW (1936) The cytoplasmic inclusion in the spermatogenesis of man. Q J Microsc Sci 78:1–33
Gottlieb W, Meizel S (1987) Biochemical studies of metalloendoproteinase activity in the spermatozoa of three mammalian species. J Androl 8:14–24
Green DPL (1978) The activation of proteolysis in the acrosome reaction of guinea pig sperm. J Cell Sci 32:153–164
Haraguchi CM, Mabuchi T, Hirata S, Shoda T, Yamada AT, Hoshi K, Yokota S (2003) Spatiotemporal changes of levels of a moonlighting protein, phospholipids hydroperoxide glutathione peroxidase, in subcellular compartments during the spermatogenesis in the rat testis. Biol Reprod 102 (in press)
Hartree EF, Strivastava PN (1969) The chemical composition of the acrosomes of rat spermatozoa. J Reprod Fertil 9:47–54
Igdoura SA, Morales CR, Hermo L (1995) Differential expression of cathepsins B and D in testis and epididymis of adult rats. J Histochem Cytochem 43:545–557
Jeffery C (1999) Moonlighting proteins. Trends Biochem Sci 24:8–11
Jin Q-S, Kamata M, Garcia del Saz E, Seguchi H (1995) Ultracytochemical study of trimetaphosphatase activity during acrosomal formation in the mouse testis. Histol Histopathol 10:681–689
Kominami, E, Tsukahara T, Bando Y, Katunuma N (1985) Distribution of cathepsins B and H in rat tissues and peripheral blood cells. J Biochem 98:87–93
Kryvi H, Graebner I (1975) Acrosome formation and the centriolar complex in the spermatozoa of Sabella penicillum (Polychaeta). An electron microscopical study. Cell Tissue Res 161:47–53
Lalli M, Clermont Y (1981) Structural changes of the head components of the rat spermatid during late spermatogenesis. Am J Anat 160:419–434
Lui CW, Meizel S (1979) Further evidence in support of a role for hamster sperm hydrolytic enzymes in the acrosome reaction. J Exp Zool 207:173–186
Martínez-Menárguez JA, Geuze HJ, Ballesta J (1996) Evidence for a nonlysosomal origin of the acrosome. J Histochem Cytochem 44:313–320
McDonald JK, Kadkhodayan S (1988) Cathepsin L: a latent proteinase in guinea pig sperm. Biochem Biophys Res Commun 151:827–835
McDonald JK, Culbertson JT, Owers NO (1993) Identification and localization of a novel cathepsin S-like proteinase in guinea pig spermatozoa. Arch Biochem Biophys 305:1–8
McRorie RA, Turner RB, Bradford MM, Williams WL (1976) Acrolysin, the aminoproteinase catalyzing the initial conversion of proacrosin to acrosin in mammalian fertilization. Biochem Biophys Res Commun 71:492–498
Meizel S, Lui CW (1976) Evidence for a rol of a trypsin-like enzyme in the hamster sperm acrosome reaction (1). J Exp Zool 195:137–144
Moreno RD, Ramalho-Santos J, Chan EKL, Wessel GM, Schatten G (2000) The Golgi apparatus segregates from the lysosomal/acrosomal vesicle during Rhesus spermiogenesis: structural alterations. Dev Biol 219:334–349
Perreault SD, Zirkin BR, Rogers BJ (1982) Effect of trypsin inhibitors on acrosome reaction of guinea pig spermatozoa. Biol Reprod 26:343–351
Polakoski KL, McRorie RA (1973) Boar acrosin. II. Classification, inhibition and specificity studies of a proteinase from sperm acrosomes. J Biol Chem 248:8183–8188
Robinson JM, Vandre DD (2001) Antigen retrieval in cells and tissues: enhancement with sodium dodecyl sulfate. Histochem Cell Biol 116:119–130
Russel LD, Ettlin RA, Sinha Hikim AM, Clegg ED (1990) Histological and histopathological evaluation of the testis. Cache River Press, Clearwater, pp 41–119
Sandoz D (1970) Evolution des ultrastructures au cours de la formation de l'acrosome du seprmatozoide chez la souris. J Microsc 9:535–558
Schollmeister JE (1986) Identification of calpain II in porcine sperm. Biol Reprod 34:721–731
Scott RP, Ninjoor V, Srivastava PN (1987) Isolation and characterization of cathepsin B from rabbit testis. J Reprod Fertil 79:67–74
Srivastava PN, Ninjoor V (1982) Isolation of rabbit testicular cathepsin D and its role in the activation of proacrosin. Biochem Biophys Res Commun 109:63–69
Stambaugh RL, Buckley J (1969) Identification and subcellular location of the enzymes effecting penetration of the zona pellucida by rabbit spermatozoa. J Reprod Fertil 19:423–432
Susi RF, Leblond CP, Clermont Y (1971) Changes in the Golgi apparatus during spermatogenesis in the rat. Am J Anat 130:251–267
Talbot P, Dicarlantonio G (1985) Cytochemical localization of dipeptidyl peptidase II (DPP-II) in mature guinea pig sperm. J Histochem Cytochem 33:1169–1172
Tanaka Y, Tanaka R, Himeno M (2000) Lysosomal cystein protease, cathepsin H, is targeted to lysosomes by the mannose 6-phosphate-independent system in rat hepatocytes. Biol Pharm Bull 23:805–809
Tang XM, Lalli MF, Clermont Y (1982) A cytochemical study of the Golgi apparatus of the spermatid during spermiogenesis in the rat. Am J Anat 163:283–294
Thorne-Tjomsland G, Clermont Y, Hermo L (1988) Contribution of the Golgi apparatus components to the formation of the acrosomic system and chromatoid body in rat spermatids. Anat Rec 221:591–598
Tulsiani DRP, Yoshida-Komiya H, Araki Y (1997) Mammalian fertilization: a carbohydrate mediated event. Biol Reprod 57:487–494
Tulsiani DRP, Abou-Haila A, Loeser CR, Pereira BM (1998) The biological and functional significance of the sperm acrosome and acrosomal enzyme in mammalian fertilization. Exp Cell Res 240:151–164
Wasserman PM (1995) Mammalian fertilization: egg and sperm (glyco) protein that support gamete adhesion. Am J Reprod Immunol 33:253–258
Yanagimachi R (1994) Mammalian fertilization. In: Knobil E, Neill J (eds) The physiology of reproduction. Raven, New York, pp 189–317
Yokota S, Kato K (1987) Immunocytochemical localization of cathepsins B and H in rat liver. Histochemistry 88:97–103
Yokota S, Tsuji H, Kato K (1985) Immunocytochemical localization of cathepsin D in lysosomes of cortical collecting tubule cells of the rat kidney. J Histochem Cytochem 33:191–200
Yokota S, Tsuji H, Kato K (1986) Immunocytochemical localization of cathepsin H in rat kidney. Light and electron microscopic study. Histochemistry 85:223–230
Yokota S, Nishimura I, Kato K (1988) Localization of cathepsin L in rat kidney revealed by immunoenzyme and immunogold techniques. Histochemistry 90:277–283
Yokota S, Himeno M, Roth J, Brada D, Kato K (1993) Formation of autophagosomes during degradation of excess peroxisomes induced by di-(2-ethylhexyl)-phthalate treatment. II. Immunocytochemical analysis of early and late autophagosomes. Eur J Cell Biol 62:372–383
Yotsumoto H, Sato S, Shibuya M (1984) Localization of angiotensin converting enzyme (dipeptidyl carboxypeptidase) in swine sperm by immunofluorescence. Life Sci 35:1257–1261
Zaneveld LJ, Polakoski KL, Schumacher GFB (1975) The proteolytic enzyme systems of mammalian genital tract secretions and spermatozoa. In: Lorand L (ed) Protease and biological control. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, pp 683–706
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haraguchi, C.M., Ishido, K., Kominami, E. et al. Expression of cathepsin H in differentiating rat spermatids: immunoelectron microscopic study. Histochem Cell Biol 120, 63–71 (2003). https://doi.org/10.1007/s00418-003-0545-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-003-0545-0