Abstract
Purpose
To investigate the efficacy and safety of loading phase treatment with 3 monthly intravitreal injections of faricimab for neovascular age-related macular degeneration (nAMD).
Methods
We retrospectively analyzed 16-week outcomes of 40 consecutive eyes of 38 patients with treatment-naïve nAMD. Three monthly injections of faricimab were administered to all eyes as a loading phase treatment. Best-corrected visual acuity (BCVA), foveal thickness, central choroidal thickness (CCT), and dry macula achievement were all assessed every 4 weeks. Moreover, the regression of polypoidal lesions was evaluated after the loading phase.
Results
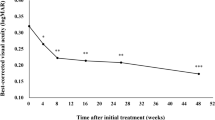
BCVA was 0.33 ± 0.41 at baseline and showed significant improvement to 0.22 ± 0.36 at week 16 (P < 0.01). Foveal thickness was 278 ± 116 µm at baseline, decreasing significantly to 173 ± 48 µm at week 16 (P < 0.01). CCT was 214 ± 98 µm at baseline, decreasing significantly to 192 ± 89 µm at week 16 (P < 0.01). Dry macula was achieved in 31 eyes (79.5%) at week 16. Indocyanine green angiography after the loading phase revealed complete regression of polypoidal lesions in 11 of 18 eyes (61.1%) with polypoidal lesions. One eye (2.5%) developed vitritis without visual loss at week 16.
Conclusion
Loading phase treatment with intravitreal faricimab appears to generally be safe and effective for improving visual acuity and reducing exudative changes in eyes with nAMD.





Similar content being viewed by others
References
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Group MS (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355:1419–1431. https://doi.org/10.1056/NEJMoa054481
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S, Group AS (2006) Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 355:1432–1444. https://doi.org/10.1056/NEJMoa062655
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, Group AS (2009) Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 116:57-65 e55. https://doi.org/10.1016/j.ophtha.2008.10.018
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U, View, Groups VS (2012) Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 119:2537–2548. https://doi.org/10.1016/j.ophtha.2012.09.006
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, Ho AC, Ogura Y, Simader C, Jaffe GJ, Slakter JS, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Sowade O, Zeitz O, Norenberg C, Sandbrink R, Heier JS (2014) Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology 121:193–201. https://doi.org/10.1016/j.ophtha.2013.08.011
Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, Gomes AV, Warburton J, Weichselberger A, Holz FG, Hawk, Investigators HS (2020) HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 127:72–84. https://doi.org/10.1016/j.ophtha.2019.04.017
Dugel PU, Singh RP, Koh A, Ogura Y, Weissgerber G, Gedif K, Jaffe GJ, Tadayoni R, Schmidt-Erfurth U, Holz FG (2021) HAWK and HARRIER: ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 128:89–99. https://doi.org/10.1016/j.ophtha.2020.06.028
Ferrara N, Damico L, Shams N, Lowman H, Kim R (2006) Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 26:859–870. https://doi.org/10.1097/01.iae.0000242842.14624.e7
Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, Pyles EA, Yancopoulos GD, Stahl N, Wiegand SJ (2012) Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 15:171–185. https://doi.org/10.1007/s10456-011-9249-6
Nguyen QD, Das A, Do DV, Dugel PU, Gomes A, Holz FG, Koh A, Pan CK, Sepah YJ, Patel N, MacLeod H, Maurer P (2020) Brolucizumab: evolution through preclinical and clinical studies and the implications for the management of neovascular age-related macular degeneration. Ophthalmology 127:963–976. https://doi.org/10.1016/j.ophtha.2019.12.031
Mones J, Srivastava SK, Jaffe GJ, Tadayoni R, Albini TA, Kaiser PK, Holz FG, Korobelnik JF, Kim IK, Pruente C, Murray TG, Heier JS (2021) Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology 128:1050–1059. https://doi.org/10.1016/j.ophtha.2020.11.011
Regula JT, Lundh von Leithner P, Foxton R, Barathi VA, Cheung CM, Bo Tun SB, Wey YS, Iwata D, Dostalek M, Moelleken J, Stubenrauch KG, Nogoceke E, Widmer G, Strassburger P, Koss MJ, Klein C, Shima DT, Hartmann G (2016) Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol Med 8:1265–1288. https://doi.org/10.15252/emmm.201505889
Heier JS, Khanani AM, Quezada Ruiz C, Basu K, Ferrone PJ, Brittain C, Figueroa MS, Lin H, Holz FG, Patel V, Lai TYY, Silverman D, Regillo C, Swaminathan B, Viola F, Cheung CMG, Wong TY, Tenaya IL (2022) Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet 399:729–740. https://doi.org/10.1016/S0140-6736(22)00010-1
Cheung CMG, Guymer RH, Demetriades AM, Margaron P, Quezada Ruiz C, Silverman D, Ives J, Basu K, Souverain A, Yang M, Lin H (2022) Faricimab in neovascular age related macular degeneration (nAMD): efficacy, safety, and durability through week 48 in the phase 3 TENAYA and LUCERNE trials. The 22nd EURETINA Congress
Ohji M, Okada AA, Sasaki K, Moon SC, Machewitz T, Takahashi K, Investigators A (2021) Relationship between retinal fluid and visual acuity in patients with exudative age-related macular degeneration treated with intravitreal aflibercept using a treat-and-extend regimen: subgroup and post-hoc analyses from the ALTAIR study. Graefes Arch Clin Exp Ophthalmol 259:3637–3647. https://doi.org/10.1007/s00417-021-05293-y
Matsumoto H, Hoshino J, Mukai R, Nakamura K, Akiyama H (2022) One-year results of treat-and-extend regimen with intravitreal brolucizumab for treatment-naive neovascular age-related macular degeneration with type 1 macular neovascularization. Sci Rep 12:8195. https://doi.org/10.1038/s41598-022-10578-1
Ohji M, Takahashi K, Okada AA, Kobayashi M, Matsuda Y, Terano Y, Investigators A (2020) Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-Week Findings from ALTAIR : A randomized controlled trial. Adv Ther 37:1173–1187. https://doi.org/10.1007/s12325-020-01236-x
Spaide RF, Jaffe GJ, Sarraf D, Freund KB, Sadda SR, Staurenghi G, Waheed NK, Chakravarthy U, Rosenfeld PJ, Holz FG, Souied EH, Cohen SY, Querques G, Ohno-Matsui K, Boyer D, Gaudric A, Blodi B, Baumal CR, Li X, Coscas GJ, Brucker A, Singerman L, Luthert P, Schmitz-Valckenberg S, Schmidt-Erfurth U, Grossniklaus HE, Wilson DJ, Guymer R, Yannuzzi LA, Chew EY, Csaky K, Mones JM, Pauleikhoff D, Tadayoni R, Fujimoto J (2020) Consensus nomenclature for reporting neovascular age-related macular degeneration data: consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology 127:616–636. https://doi.org/10.1016/j.ophtha.2019.11.004
Yanai H (2015) Statcel- the useful add-in software forms on excel, 4th edn. OMS, Tokyo
Matsumoto H, Morimoto M, Mimura K, Ito A, Akiyama H (2018) Treat-and-extend regimen with aflibercept for neovascular age-related macular degeneration: efficacy and macular atrophy development. Ophthalmol Retina 2:462–468. https://doi.org/10.1016/j.oret.2017.09.002
Cho JH, Ryoo NK, Cho KH, Park SJ, Park KH, Woo SJ (2016) Incidence rate of massive submacular hemorrhage and its risk factors in polypoidal choroidal vasculopathy. Am J Ophthalmol 169:79–88. https://doi.org/10.1016/j.ajo.2016.06.014
Morimoto M, Matsumoto H, Mimura K, Akiyama H (2017) Two-year results of a treat-and-extend regimen with aflibercept for polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 255:1891–1897. https://doi.org/10.1007/s00417-017-3718-6
Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H, Lai TY, Pilz S, Ruamviboonsuk P, Tokaji E, Weisberger A, Lim TH (2012) EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina 32:1453–1464. https://doi.org/10.1097/IAE.0b013e31824f91e8
Gomi F, Oshima Y, Mori R, Kano M, Saito M, Yamashita A, Iwata E, Maruko R, Fujisan Study G (2015) Initial versus delayed photodynamic therapy in combination with ranibizumab for treatment of polypoidal choroidal vasculopathy: the Fujisan study. Retina 35:1569–1576. https://doi.org/10.1097/IAE.0000000000000526
Koh A, Lai TYY, Takahashi K, Wong TY, Chen LJ, Ruamviboonsuk P, Tan CS, Feller C, Margaron P, Lim TH, Lee WK, group EIs (2017) Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: a randomized clinical trial. JAMA Ophthalmol 135:1206–1213. https://doi.org/10.1001/jamaophthalmol.2017.4030
Yamamoto A, Okada AA, Kano M, Koizumi H, Saito M, Maruko I, Sekiryu T, Iida T (2015) One-year results of intravitreal aflibercept for polypoidal choroidal vasculopathy. Ophthalmology 122:1866–1872. https://doi.org/10.1016/j.ophtha.2015.05.024
Kikushima W, Sakurada Y, Sugiyama A, Tanabe N, Kume A, Iijima H (2017) Comparison of initial treatment between 3-monthly intravitreal aflibercept monotherapy and combined photodynamic therapy with single intravitreal aflibercept for polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 255:311–316. https://doi.org/10.1007/s00417-016-3467-y
Fukuda Y, Sakurada Y, Matsubara M, Hasebe Y, Sugiyama A, Kikushima W, Kashiwagi K (2021) Comparison of outcomes between 3 monthly brolucizumab and aflibercept injections for polypoidal choroidal vasculopathy. Biomedicines 9:1164. https://doi.org/10.3390/biomedicines9091164
Matsumoto H, Hoshino J, Mukai R, Nakamura K, Akiyama H (2021) Short-term outcomes of intravitreal brolucizumab for treatment-naive neovascular age-related macular degeneration with type 1 choroidal neovascularization including polypoidal choroidal vasculopathy. Sci Rep 11:6759. https://doi.org/10.1038/s41598-021-86014-7
Ito A, Maruyama-Inoue M, Kitajima Y, Ikeda S, Inoue T, Kadonosono K (2022) One-year outcomes of intravitreal brolucizumab injections in patients with polypoidal choroidal vasculopathy. Sci Rep 12:7987. https://doi.org/10.1038/s41598-022-12216-2
Tanaka K, Koizumi H, Tamashiro T, Itagaki K, Nakayama M, Maruko I, Wakugawa S, Terao N, Onoe H, Wakatsuki Y, Kasai A, Ogasawara M, Shintake H, Sugano Y, Yamamoto A, Kataoka K, Hasegawa T, Izumi T, Kawai M, Maruko R, Sekiryu T, Okada AA, Iida T, Mori R (2022) Short-term results for brolucizumab in treatment-naive neovascular age-related macular degeneration: a Japanese multicenter study. Jpn J Ophthalmol 66:379–385. https://doi.org/10.1007/s10384-022-00922-3
Hoshino J, Matsumoto H, Mukai R, Nakamura K, Akiyama H (2022) Intravitreal aflibercept versus brolucizumab for treatment-naive neovascular age-related macular degeneration with type 1 macular neovascularization: comparison of short-term outcomes. Ophthalmologica 245:413–420. https://doi.org/10.1159/000526044
Koizumi H, Kano M, Yamamoto A, Saito M, Maruko I, Sekiryu T, Okada AA, Iida T (2016) Subfoveal choroidal thickness during aflibercept therapy for neovascular age-related macular degeneration: twelve-month results. Ophthalmology 123:617–624. https://doi.org/10.1016/j.ophtha.2015.10.039
Yamazaki T, Koizumi H, Yamagishi T, Kinoshita S (2012) Subfoveal choroidal thickness after ranibizumab therapy for neovascular age-related macular degeneration: 12-month results. Ophthalmology 119:1621–1627. https://doi.org/10.1016/j.ophtha.2012.02.022
Koizumi H, Kano M, Yamamoto A, Saito M, Maruko I, Kawasaki R, Sekiryu T, Okada AA, Iida T (2015) Short-term changes in choroidal thickness after aflibercept therapy for neovascular age-related macular degeneration. Am J Ophthalmol 159:627–633. https://doi.org/10.1016/j.ajo.2014.12.025
Tamashiro T, Tanaka K, Itagaki K, Nakayama M, Maruko I, Wakugawa S, Terao N, Onoe H, Wakatsuki Y, Ogasawara M, Sugano Y, Yamamoto A, Kataoka K, Izumi T, Kawai M, Mori R, Sekiryu T, Okada AA, Iida T, Koizumi H, Japan AMDRC (2022) Subfoveal choroidal thickness after brolucizumab therapy for neovascular age-related macular degeneration: a short-term multicenter study. Graefes Arch Clin Exp Ophthalmol 260:1857–1865
Angermann R, Huber AL, Nowosielski Y, Salcher S, Gasser T, Seifarth C, Kralinger MT, Zehetner C (2022) Changes in systemic levels of vascular endothelial growth factor after intravitreal injection of aflibercept or brolucizumab for neovascular age-related macular degeneration. Retina 42:503–510. https://doi.org/10.1097/IAE.0000000000003344
Chugai Pharmaceutical Co., Ltd (2022) Faricimab CTD (in Japanese): 2.7.2.2.4.3.3. Pharmacodynamics. Available at https://www.pmda.go.jp/drugs/2022/P20220406001/index.html. Accessed 13 Jan 2023
Author information
Authors and Affiliations
Contributions
The authors were involved in the following aspects of the study: design and conduct (H.M.), collection of the data (J.H., K.N., T.N.), management (H.M.), analysis (J.H., H.M.), interpretation (H.M.), preparation of the article (J.H., H.M.), and review and final approval of the manuscript for submission (H.A.).
Corresponding author
Ethics declarations
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board of Gunma University Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matsumoto, H., Hoshino, J., Nakamura, K. et al. Short-term outcomes of intravitreal faricimab for treatment-naïve neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 261, 2945–2952 (2023). https://doi.org/10.1007/s00417-023-06116-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06116-y