Abstract
Purpose
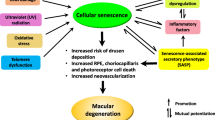
Cellular senescence is a state of permanent growth arrest whereby a cell reaches its replicative limit. However, senescence can also be triggered prematurely in certain stressors including radiation, oxidative stress, and chemotherapy. This stress-induced senescence has been studied in the context of promoting inflammation, tumor development, and several chronic degenerative diseases of aging. Emerging research has elucidated the role of senescence in various ocular diseases.
Methods
The literature search was performed using PubMed with using the query (senescence OR aging) AND (eye disease OR ocular disease OR ophthalmic disease OR cornea OR glaucoma OR cataract OR retina) on October 20th, 2022. No time restriction was proposed. Articles were excluded if they were not referenced in English.
Results
Overall, 51 articles regarding senescence and ocular diseases were found and summarized in this study. Several signaling pathways have been implicated in the development of senescence. Currently, senescence has been linked to various corneal and retinal pathologies, as well as cataract and glaucoma. Given the number of pathologies, senolytics, which are small molecules with the ability to selective targeting of senescent cells, can be used as therapeutic or prophylactic agents.
Conclusions
Senescence has been shown to underlie the pathogenesis of numerous ocular diseases. The overall literature on senescence and ocular disease is growing rapidly. There is an ongoing debate whether or not cellular senescence detected in experiments contributes in a significant way to diseases. Research on understanding the mechanism of senescence from ocular cells and tissues is just beginning. Multiple animal models are required to test potential senolytics. Currently, no studies exist to date which have demonstrated the benefits of senolytic therapies in human studies.
Similar content being viewed by others
References
Shay JW, Wright WE (2000) Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol 1:72–76
Levy MZ, Allsopp RC, Futcher AB, Greider CW, Harley CB (1992) Telomere end-replication problem and cell aging. J Mol Biol 225:951–960
Papaconstantinou J (2019) The role of signaling pathways of inflammation and oxidative stress in development of senescence and aging phenotypes in cardiovascular disease. Cells 8:1383
Abate M, Festa A, Falco M, Lombardi A, Luce A, Grimaldi A, Zappavigna S, Sperlongano P, Irace C, Caraglia M (2020) Mitochondria as playmakers of apoptosis, autophagy and senescence. Seminars in cell & developmental biology. Elsevier, 139–153
Aratani S, Tagawa M, Nagasaka S, Sakai Y, Shimizu A, Tsuruoka S (2018) Radiation-induced premature cellular senescence involved in glomerular diseases in rats. Sci Rep 8:1–12
Patel PL, Suram A, Mirani N, Bischof O, Herbig U (2016) Derepression of hTERT gene expression promotes escape from oncogene-induced cellular senescence. Proc Natl Acad Sci 113:E5024–E5033
Pazolli E, Alspach E, Milczarek A, Prior J, Piwnica-Worms D, Stewart SA (2012) Chromatin remodeling underlies the senescence-associated secretory phenotype of tumor stromal fibroblasts that supports cancer progression regulation of stroma-derived OPN. Can Res 72:2251–2261
García-Prat L, Martínez-Vicente M, Perdiguero E, Ortet L, Rodríguez-Ubreva J, Rebollo E, Ruiz-Bonilla V, Gutarra S, Ballestar E, Serrano AL (2016) Autophagy maintains stemness by preventing senescence. Nature 529:37–42
Mikuła-Pietrasik J, Niklas A, Uruski P, Tykarski A, Książek K (2020) Mechanisms and significance of therapy-induced and spontaneous senescence of cancer cells. Cell Mol Life Sci 77:213–229
Wang B, Kohli J, Demaria M (2020) Senescent cells in cancer therapy: friends or foes? Trends Cancer 6:838–857
Faget DV, Ren Q, Stewart SA (2019) Unmasking senescence: context-dependent effects of SASP in cancer. Nat Rev Cancer 19:439–453
Muñoz-Espín D, Cañamero M, Maraver A, Gómez-López G, Contreras J, Murillo-Cuesta S, Rodríguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M (2013) Programmed cell senescence during mammalian embryonic development. Cell 155:1104–1118
Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge R-M, Vijg J, Van Steeg H, Dollé ME (2014) An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31:722–733
Childs BG, Durik M, Baker DJ, Van Deursen JM (2015) Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 21:1424–1435
Rahman I, Bagchi D (2013) Inflammation, advancing age and nutrition: research and clinical interventions. Academic Press
Williams AB, Schumacher B (2016) p53 in the DNA-damage-repair process. Cold Spring Harb Perspect Med 6:a026070
Spallarossa P, Altieri P, Aloi C, Garibaldi S, Barisione C, Ghigliotti G, Fugazza G, Barsotti A, Brunelli C (2009) Doxorubicin induces senescence or apoptosis in rat neonatal cardiomyocytes by regulating the expression levels of the telomere binding factors 1 and 2. Am J Physiol-Heart Circ Physiol 297:H2169–H2181
Vicencio Bustamante JM, Galluzzi L, Tajeddine N, Ortiz C, Criollo Céspedes A, Tasdemir E, Morselli E, Ben Younes A, Maiuri MC, Lavandero González S (2008) Senescence, apoptosis or autophagy? When a damaged cell must decide its path-a mini-review
Giacinti C, Giordano A (2006) RB and cell cycle progression. Oncogene 25:5220–5227
Kumari R, Jat P (2021) Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol 9:645593
Aliouat-Denis C-M, Dendouga N, Van den Wyngaert I, Goehlmann H, Steller U, Van De Weyer I, Van Slycken N, Andries L, Kass S, Luyten W (2005) p53-independent regulation of p21Waf1/Cip1 expression and senescence by Chk2. Mol Cancer Res 3:627–634
González-Gualda E, Baker AG, Fruk L, Muñoz-Espín D (2021) A guide to assessing cellular senescence in vitro and in vivo. FEBS J 288:56–80
Stein GH, Drullinger LF, Soulard A, Dulić V (1999) Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol 19:2109–2117
Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC (1996) Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci 93:13742–13747
Palafox M, Monserrat L, Bellet M, Villacampa G, Gonzalez-Perez A, Oliveira M, Brasó-Maristany F, Ibrahimi N, Kannan S, Mina L (2022) High p16 expression and heterozygous RB1 loss are biomarkers for CDK4/6 inhibitor resistance in ER+ breast cancer. Nat Commun 13:1–20
Liu D, Xu Y (2011) p53, oxidative stress, and aging. Antioxid Redox Signal 15:1669–1678
Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM (2004) Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIP1, but not p16INK4a. Mol Cell 14:501–513
Sun P, Yoshizuka N, New L, Moser BA, Li Y, Liao R, Xie C, Chen J, Deng Q, Yamout M (2007) PRAK is essential for ras-induced senescence and tumor suppression. Cell 128:295–308
Wang Z, Wei D, Xiao H (2013) Methods of cellular senescence induction using oxidative stress. Biological aging. Springer, 135–144
Blander G, De Oliveira RM, Conboy CM, Haigis M, Guarente L (2003) Superoxide dismutase 1 knock-down induces senescence in human fibroblasts. J Biol Chem 278:38966–38969
Macip S, Igarashi M, Fang L, Chen A, Pan Z-Q, Lee SW, Aaronson SA (2002) Inhibition of p21-mediated ROS accumulation can rescue p21-induced senescence. EMBO J 21:2180–2188
Liao N, Shi Y, Zhang C, Zheng Y, Wang Y, Zhao B, Zeng Y, Liu X, Liu J (2019) Antioxidants inhibit cell senescence and preserve stemness of adipose tissue-derived stem cells by reducing ROS generation during long-term in vitro expansion. Stem Cell Res Ther 10:1–11
Petrova NV, Velichko AK, Razin SV, Kantidze OL (2016) Small molecule compounds that induce cellular senescence. Aging Cell 15:999–1017
Jung SH, Hwang HJ, Kang D, Park HA, Lee HC, Jeong D, Lee K, Park HJ, Ko Y-G, Lee J-S (2019) mTOR kinase leads to PTEN-loss-induced cellular senescence by phosphorylating p53. Oncogene 38:1639–1650
Xu Y, Li N, Xiang R, Sun P (2014) Emerging roles of the p38 MAPK and PI3K/AKT/mTOR pathways in oncogene-induced senescence. Trends Biochem Sci 39:268–276
Son HN, Chi HNQ, Chung DC, Long LT (2019) Morphological changes during replicative senescence in bovine ovarian granulosa cells. Cell Cycle 18:1490–1497
Hernandez-Segura A, Nehme J, Demaria M (2018) Hallmarks of cellular senescence. Trends Cell Biol 28:436–453
Ryu S, Oh Y, Park S (2007) Failure of stress-induced downregulation of Bcl-2 contributes to apoptosis resistance in senescent human diploid fibroblasts. Cell Death Differ 14:1020–1028
Beck J, Horikawa I, Harris C (2020) Cellular senescence: mechanisms, morphology, and mouse models. Vet Pathol 57:747–757
Citrin DE, Shankavaram U, Horton JA, Shield W III, Zhao S, Asano H, White A, Sowers A, Thetford A, Chung EJ (2013) Role of type II pneumocyte senescence in radiation-induced lung fibrosis. J Natl Cancer Inst 105:1474–1484
da Silva PF, Ogrodnik M, Kucheryavenko O, Glibert J, Miwa S, Cameron K, Ishaq A, Saretzki G, Nagaraja-Grellscheid S, Nelson G (2019) The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell 18:e12848
Van Deursen JM (2014) The role of senescent cells in ageing. Nature 509:439–446
Ohanna M, Giuliano S, Bonet C, Imbert V, Hofman V, Zangari J, Bille K, Robert C, Bressac-de Paillerets B, Hofman P (2011) Senescent cells develop a PARP-1 and nuclear factor-κB-associated secretome (PNAS). Genes Dev 25:1245–1261
Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A (2016) Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab 23:303–314
Hoare M, Ito Y, Kang T-W, Weekes MP, Matheson NJ, Patten DA, Shetty S, Parry AJ, Menon S, Salama R (2016) NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat Cell Biol 18:979–992
Herranz N, Gallage S, Mellone M, Wuestefeld T, Klotz S, Hanley CJ, Raguz S, Acosta JC, Innes AJ, Banito A (2015) mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol 17:1205–1217
Bent EH, Gilbert LA, Hemann MT (2016) A senescence secretory switch mediated by PI3K/AKT/mTOR activation controls chemoprotective endothelial secretory responses. Genes Dev 30:1811–1821
Rajagopalan S, Long EO (2012) Cellular senescence induced by CD158d reprograms natural killer cells to promote vascular remodeling. Proc Natl Acad Sci 109:20596–20601
Adams PD (2009) Healing and hurting: molecular mechanisms, functions, and pathologies of cellular senescence. Mol Cell 36:2–14
Cosme-Blanco W, Shen MF, Lazar AJ, Pathak S, Lozano G, Multani AS, Chang S (2007) Telomere dysfunction suppresses spontaneous tumorigenesis in vivo by initiating p53-dependent cellular senescence. EMBO Rep 8:497–503
Jun J-I, Lau LF (2010) The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 12:676–685
Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW (2008) Senescence of activated stellate cells limits liver fibrosis. Cell 134:657–667
Shimizu I, Yoshida Y, Katsuno T, Tateno K, Okada S, Moriya J, Yokoyama M, Nojima A, Ito T, Zechner R (2012) p53-induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure. Cell Metab 15:51–64
Thangavel C, Dean JL, Ertel A, Knudsen KE, Aldaz CM, Witkiewicz AK, Clarke R, Knudsen ES (2011) Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer 18:333–345
Zhou X, Perez F, Han K, Jurivich DA (2006) Clonal senescence alters endothelial ICAM-1 function. Mech Ageing Dev 127:779–785. https://doi.org/10.1016/j.mad.2006.07.003
Chilosi M, Carloni A, Rossi A, Poletti V (2013) Premature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema. Translational research : the journal of laboratory and clinical medicine 162:156–173. https://doi.org/10.1016/j.trsl.2013.06.004
Martin JA, Brown TD, Heiner AD, Buckwalter JA (2004) Chondrocyte senescence, joint loading and osteoarthritis. Clinical orthopaedics and related research: S96–103 https://doi.org/10.1097/01.blo.0000143818.74887.b1
Liton PB, Challa P, Stinnett S, Luna C, Epstein DL, Gonzalez P (2005) Cellular senescence in the glaucomatous outflow pathway. Exp Gerontol 40:745–748. https://doi.org/10.1016/j.exger.2005.06.005
Sone H, Kagawa Y (2005) Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia 48:58–67. https://doi.org/10.1007/s00125-004-1605-2
Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F, Komuro I (2009) A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 15:1082–1087. https://doi.org/10.1038/nm.2014
Braun H, Schmidt BM, Raiss M, Baisantry A, Mircea-Constantin D, Wang S, Gross ML, Serrano M, Schmitt R, Melk A (2012) Cellular senescence limits regenerative capacity and allograft survival. J Am Soc Nephrol 23:1467–1473. https://doi.org/10.1681/asn.2011100967
Melk A, Schmidt BM, Braun H, Vongwiwatana A, Urmson J, Zhu LF, Rayner D, Halloran PF (2009) Effects of donor age and cell senescence on kidney allograft survival. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg 9:114–123. https://doi.org/10.1111/j.1600-6143.2008.02500.x
Li ZY, Chen ZL, Zhang T, Wei C, Shi WY (2016) TGF-β and NF-κB signaling pathway crosstalk potentiates corneal epithelial senescence through an RNA stress response. Aging 8:2337–2354. https://doi.org/10.18632/aging.101050
Bae Y, Hwang JS, Shin YJ (2021) miR-30c-1 encourages human corneal endothelial cells to regenerate through ameliorating senescence. Aging 13:9348–9372. https://doi.org/10.18632/aging.202719
De Paiva CS, Volpe EA, Gandhi NB, Zhang X, Zheng X, Pitcher JD 3rd, Farley WJ, Stern ME, Niederkorn JY, Li DQ, Flavell RA, Pflugfelder SC (2011) Disruption of TGF-β signaling improves ocular surface epithelial disease in experimental autoimmune keratoconjunctivitis sicca. PloS One 6:e29017. https://doi.org/10.1371/journal.pone.0029017
Ogawa Y, Yamazaki K, Kuwana M, Mashima Y, Nakamura Y, Ishida S, Toda I, Oguchi Y, Tsubota K, Okamoto S, Kawakami Y (2001) A significant role of stromal fibroblasts in rapidly progressive dry eye in patients with chronic GVHD. Invest Ophthalmol Vis Sci 42:111–119
Leonardi A, Di Stefano A, Motterle L, Zavan B, Abatangelo G, Brun P (2011) Transforming growth factor-β/Smad - signalling pathway and conjunctival remodelling in vernal keratoconjunctivitis. Clin Exp Allergy : J Br Soc Allergy Clin Immunol 41:52–60. https://doi.org/10.1111/j.1365-2222.2010.03626.x
Kria L, Ohira A, Amemiya T (1996) Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-beta and tumor necrosis factor-alpha in the pterygium. Acta Histochem 98:195–201. https://doi.org/10.1016/s0065-1281(96)80038-9
Saika S (2006) TGFbeta pathobiology in the eye. Laboratory investigation; a J Tech Methods Pathol 86:106–115 https://doi.org/10.1038/labinvest.3700375
Benito MJ, Calder V, Corrales RM, García-Vázquez C, Narayanan S, Herreras JM, Stern ME, Calonge M, Enríquez-de-Salamanca A (2013) Effect of TGF-β on ocular surface epithelial cells. Exp Eye Res 107:88–100. https://doi.org/10.1016/j.exer.2012.11.017
Mimura T, Joyce NC (2006) Replication competence and senescence in central and peripheral human corneal endothelium. Invest Ophthalmol Vis Sci 47:1387–1396
Wang Y, Zang X, Wang Y, Chen P (2012) High expression of p16INK4a and low expression of Bmi1 are associated with endothelial cellular senescence in the human cornea. Mol Vis 18:803–815
Pathai S, Lawn SD, Shiels PG, Weiss HA, Cook C, Wood R, Gilbert CE (2013) Corneal endothelial cells provide evidence of accelerated cellular senescence associated with HIV infection: a case-control study. PloS One 8:e57422. https://doi.org/10.1371/journal.pone.0057422
Faragher R, Mulholland B, Tuft S, Sandeman S, Khaw P (1997) Aging and the cornea. Br J Ophthalmol 81:814–817
Gipson IK (2013) Age-related changes and diseases of the ocular surface and cornea. Investig Ophthalmol Vis Sci 54:ORSF48-ORSF53
Darlington JK, Adrean SD, Schwab IR (2006) Trends of penetrating keratoplasty in the United States from 1980 to 2004. Ophthalmology 113:2171–2175. https://doi.org/10.1016/j.ophtha.2006.06.034
Matthaei M, Meng H, Meeker AK, Eberhart CG, Jun AS (2012) Endothelial Cdkn1a (p21) overexpression and accelerated senescence in a mouse model of Fuchs endothelial corneal dystrophy. Invest Ophthalmol Vis Sci 53:6718–6727. https://doi.org/10.1167/iovs.12-9669
Matthaei M, Hu J, Kallay L, Eberhart CG, Cursiefen C, Qian J, Lackner EM, Jun AS (2014) Endothelial cell microRNA expression in human late-onset Fuchs’ dystrophy. Invest Ophthalmol Vis Sci 55:216–225. https://doi.org/10.1167/iovs.13-12689
Zhao X, Wang Y, Wang Y, Li S, Chen P (2016) Oxidative stress and premature senescence in corneal endothelium following penetrating keratoplasty in an animal model. BMC Ophthalmol 16:16. https://doi.org/10.1186/s12886-016-0192-6
Nassiri N, Eslani M, Panahi N, Mehravaran S, Ziaei A, Djalilian AR (2013) Ocular graft versus host disease following allogeneic stem cell transplantation: a review of current knowledge and recommendations. J Ophthalmic Vis Res 8:351
Tabbara KF, Al-Ghamdi A, Al-Mohareb F, Ayas M, Chaudhri N, Al-Sharif F, Al-Zahrani H, Mohammed SY, Nassar A, Aljurf M (2009) Ocular findings after allogeneic hematopoietic stem cell transplantation. Ophthalmology 116:1624–1629
Yamane M, Sato S, Shimizu E, Shibata S, Hayano M, Yaguchi T, Kamijuku H, Ogawa M, Suzuki T, Mukai S (2020) Senescence-associated secretory phenotype promotes chronic ocular graft-vs-host disease in mice and humans. FASEB J 34:10778–10800
Kawai M, Ogawa Y, Shimmura S, Ohta S, Suzuki T, Kawamura N, Kuwana M, Kawakami Y, Tsubota K (2013) Expression and localization of aging markers in lacrimal gland of chronic graft-versus-host disease. Sci Rep 3:1–6
Kitazawa K, Inotmata T, Shih K, Hughes J-WB, Bozza N, Tomioka Y, Numa K, Yokoi N, Campisi J, Dana R (2022) Impact of aging on the pathophysiology of dry eye disease: a systematic review and meta-analysis. The Ocular Surface
Mimura T, Yamagami S, Usui T, Funatsu H, Mimura Y, Noma H, Honda N, Amano S (2009) Changes of conjunctivochalasis with age in a hospital-based study. Am J Ophthalmol 147(171–177):e171
Watanabe A, Yokoi N, Kinoshita S, Hino Y, Tsuchihashi Y (2004) Clinicopathologic study of conjunctivochalasis. Cornea 23:294–298
Ward SK, Wakamatsu TH, Dogru M, Ibrahim OM, Kaido M, Ogawa Y, Matsumoto Y, Igarashi A, Ishida R, Shimazaki J (2010) The role of oxidative stress and inflammation in conjunctivochalasis. Invest Ophthalmol Vis Sci 51:1994–2002
Xiang M, Mo L, Zhan Y, Wen H, Zhou H, Miao W (2019) P38-mediated cellular senescence in conjunctivochalasis fibroblasts. Invest Ophthalmol Vis Sci 60:4643–4651
Liu J, Zhang J, Zhang G, Zhou T, Zou X, Guan H, Wang Y (2021) CircMRE11A_013 binds to UBXN1 and integrates ATM activation enhancing lens epithelial cells senescence in age-related cataract. Aging 13:5383
Ivanov IV, Mappes T, Schaupp P, Lappe C, Wahl S (2018) Ultraviolet radiation oxidative stress affects eye health. J Biophotonics 11:e201700377
Asbell PA, Dualan I, Mindel J, Brocks D, Ahmad M, Epstein S (2005) Age-related cataract. The Lancet 365:599–609
Fu Q, Qin Z, Yu J, Yu Y, Tang Q, Lyu D, Zhang L, Chen Z, Yao K (2016) Effects of senescent lens epithelial cells on the severity of age-related cortical cataract in humans: a case–control study. Medicine 95
Yan Y, Yu H, Sun L, Liu H, Wang C, Wei X, Song F, Li H, Ge H, Qian H (2019) Laminin α4 overexpression in the anterior lens capsule may contribute to the senescence of human lens epithelial cells in age-related cataract. Aging 11:2699
Matsuyama M, Tanaka H, Inoko A, Goto H, Yonemura S, Kobori K, Hayashi Y, Kondo E, Itohara S, Izawa I (2013) Defect of mitotic vimentin phosphorylation causes microophthalmia and cataract via aneuploidy and senescence in lens epithelial cells*♦. J Biol Chem 288:35626–35635
Gulluni F, Prever L, Li H, Krafcikova P, Corrado I, Lo W-T, Margaria JP, Chen A, De Santis MC, Cnudde SJ (2021) PI (3, 4) P2-mediated cytokinetic abscission prevents early senescence and cataract formation. Science 374:eabk0410
Huang Y, Liu Y, Yu S, Li W, Li J, Zhao B, Hu X, Jin H (2022) Biliverdin reductase A protects lens epithelial cells against oxidative damage and cellular senescence in age-related cataract. Oxidative Med Cell Longev 2022
Caprioli J (2013) Glaucoma: a disease of early cellular senescence. Invest Ophthalmol Vis Sci 54:ORSF60-ORSF67
Balazsi A, Rootman J, Drance S, Schulzer M, Douglas G (1984) The effect of age on the nerve fiber population of the human optic nerve. Am J Ophthalmol 97:760–766
Skowronska-Krawczyk D, Zhao L, Zhu J, Weinreb RN, Cao G, Luo J, Flagg K, Patel S, Wen C, Krupa M (2015) P16INK4a upregulation mediated by SIX6 defines retinal ganglion cell pathogenesis in glaucoma. Mol Cell 59:931–940
Rocha LR, Nguyen Huu VA, Palomino La Torre C, Xu Q, Jabari M, Krawczyk M, Weinreb RN, Skowronska-Krawczyk D (2020) Early removal of senescent cells protects retinal ganglion cells loss in experimental ocular hypertension. Aging Cell 19:e13089
Coudrillier B, Tian J, Alexander S, Myers KM, Quigley HA, Nguyen TD (2012) Biomechanics of the human posterior sclera: age-and glaucoma-related changes measured using inflation testing. Invest Ophthalmol Vis Sci 53:1714–1728
Wostyn P, De Groot V, Van Dam D, Audenaert K, De Deyn PP (2013) Senescent changes in cerebrospinal fluid circulatory physiology and their role in the pathogenesis of normal-tension glaucoma. Am J Ophthalmol 156(5–14):e12
Embleton S, Hosking S, Roff Hilton E, Cunliffe I (2002) Effect of senescence on ocular blood flow in the retina, neuroretinal rim and lamina cribrosa, using scanning laser Doppler flowmetry. Eye 16:156–162
Morgan JT, Raghunathan VK, Chang Y-R, Murphy CJ, Russell P (2015) The intrinsic stiffness of human trabecular meshwork cells increases with senescence. Oncotarget 6:15362
Alice LY, Birke K, Moriniere J, Welge-Lüssen U (2010) TGF-β2 induces senescence-associated changes in human trabecular meshwork cells. Invest Ophthalmol Vis Sci 51:5718–5723
Gehrs KM, Anderson DH, Johnson LV, Hageman GS (2006) Age-related macular degeneration—emerging pathogenetic and therapeutic concepts. Ann Med 38:450–471
Zhu D, Wu J, Spee C, Ryan SJ, Hinton DR (2009) BMP4 mediates oxidative stress-induced retinal pigment epithelial cell senescence and is overexpressed in age-related macular degeneration. J Biol Chem 284:9529–9539
Liu C, Cao L, Yang S, Xu L, Liu P, Wang F, Xu D (2015) Subretinal injection of amyloid-β peptide accelerates RPE cell senescence and retinal degeneration. Int J Mol Med 35:169–176
Kaarniranta K, Kajdanek J, Morawiec J, Pawlowska E, Blasiak J (2018) PGC-1α protects RPE cells of the aging retina against oxidative stress-induced degeneration through the regulation of senescence and mitochondrial quality control. The significance for AMD pathogenesis. Int J Mol Sci 19:2317
Lee KS, Lin S, Copland DA, Dick AD, Liu J (2021) Cellular senescence in the aging retina and developments of senotherapies for age-related macular degeneration. J Neuroinflammation 18:1–17
Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O’Malley B, Spiegelman BM (1999) Activation of PPARγ coactivator-1 through transcription factor docking. Science 286:1368–1371
Blasiak J, Piechota M, Pawlowska E, Szatkowska M, Sikora E, Kaarniranta K (2017) Cellular senescence in age-related macular degeneration: can autophagy and DNA damage response play a role? Oxidative Med Cell Longev 2017
Egger A, Samardzija M, Sothilingam V, Tanimoto N, Lange C, Salatino S, Fang L, Garcia-Garrido M, Beck S, Okoniewski MJ (2012) PGC-1α determines light damage susceptibility of the murine retina. PLoS One 7:e31272
Schmidt-Erfurth U, Chong V, Loewenstein A, Larsen M, Souied E, Schlingemann R, Eldem B, Monés J, Richard G, Bandello F (2014) Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol 98:1144–1167
Mishima K, Handa JT, Aotaki-Keen A, Lutty GA, Morse LS, Hjelmeland LM (1999) Senescence-associated beta-galactosidase histochemistry for the primate eye. Invest Ophthalmol Vis Sci 40:1590–1593
López-Luppo M, Catita J, Ramos D, Navarro M, Carretero A, Mendes-Jorge L, Muñoz-Cánoves P, Rodriguez-Baeza A, Nacher V, Ruberte J (2017) Cellular senescence is associated with human retinal microaneurysm formation during aging. Invest Ophthalmol Vis Sci 58:2832–2842
Chaum E, Winborn CS, Bhattacharya S (2015) Genomic regulation of senescence and innate immunity signaling in the retinal pigment epithelium. Mamm Genome 26:210–221
Oubaha M, Miloudi K, Dejda A, Guber V, Mawambo G, Germain M-A, Bourdel G, Popovic N, Rezende FA, Kaufman RJ (2016) Senescence-associated secretory phenotype contributes to pathological angiogenesis in retinopathy. Sci Transl Med 8:362ra144–362ra144
Cabrera AP, Bhaskaran A, Xu J, Yang X, Scott HA, Mohideen U, Ghosh K (2016) Senescence increases choroidal endothelial stiffness and susceptibility to complement injury: implications for choriocapillaris loss in AMD. Invest Ophthalmol Vis Sci 57:5910–5918
Sene A, Khan AA, Cox D, Nakamura RE, Santeford A, Kim BM, Sidhu R, Onken MD, Harbour JW, Hagbi-Levi S (2013) Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell Metab 17:549–561
Lamoke F, Shaw S, Yuan J, Ananth S, Duncan M, Martin P, Bartoli M (2015) Increased oxidative and nitrative stress accelerates aging of the retinal vasculature in the diabetic retina. PLoS One 10:e0139664
Crespo-Garcia S, Tsuruda PR, Dejda A, Ryan RD, Fournier F, Chaney SY, Pilon F, Dogan T, Cagnone G, Patel P (2021) Pathological angiogenesis in retinopathy engages cellular senescence and is amenable to therapeutic elimination via BCL-xL inhibition. Cell Metab 33(818–832):e817
Thounaojam MC, Jadeja RN, Warren M, Powell FL, Raju R, Gutsaeva D, Khurana S, Martin PM, Bartoli M (2019) MicroRNA-34a (miR-34a) mediates retinal endothelial cell premature senescence through mitochondrial dysfunction and loss of antioxidant activities. Antioxidants 8:328
Rojas M, Lemtalsi T, Toque HA, Xu Z, Fulton D, Caldwell RW, Caldwell RB (2017) NOX2-induced activation of arginase and diabetes-induced retinal endothelial cell senescence. Antioxidants 6:43
Shosha E, Xu Z, Narayanan SP, Lemtalsi T, Fouda AY, Rojas M, Xing J, Fulton D, Caldwell RW, Caldwell RB (2018) Mechanisms of diabetes-induced endothelial cell senescence: role of arginase 1. Int J Mol Sci 19:1215
Merz S, Kershaw O, Petrick A, Gruber A, Klopfleisch R, Breithaupt A (2019) Tumour, but not age-associated, increase of senescence markers γH2AX and p21 in the canine eye. J Comp Pathol 173:41–48
Kirkland J, Tchkonia T (2020) Senolytic drugs: from discovery to translation. J Intern Med 288:518–536
Stefanova NA, Fursova AZ, Sarsenbaev KN, Kolosova NG (2011) Effects of Cistanche deserticola on behavior and signs of cataract and retinopathy in senescence-accelerated OXYS rats. J Ethnopharmacol 138:624–632
Wei X, Luo D, Yan Y, Yu H, Sun L, Wang C, Song F, Ge H, Qian H, Li X (2019) Kojic acid inhibits senescence of human corneal endothelial cells via NF-κB and p21 signaling pathways. Exp Eye Res 180:174–183
Gidfar S, Milani FY, Milani BY, Shen X, Eslani M, Putra I, Huvard MJ, Sagha H, Djalilian AR (2017) Rapamycin prolongs the survival of corneal epithelial cells in culture. Sci Rep 7:1–10
Sun Q, Qing W, Qi R, Zou M, Gong L, Liu Y, Li D-C (2018) Inhibition of Sumoylation alleviates oxidative stress-induced retinal pigment epithelial cell senescence and represses proinflammatory gene expression. Curr Mol Med 18:575–583
Campello L, Esteve-Rudd J, Cuenca N, Martín-Nieto J (2013) The ubiquitin–proteasome system in retinal health and disease. Mol Neurobiol 47:790–810
Zhu Y, Doornebal EJ, Pirtskhalava T, Giorgadze N, Wentworth M, Fuhrmann-Stroissnigg H, Niedernhofer LJ, Robbins PD, Tchkonia T, Kirkland JL (2017) New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging 9:955
Baar MP, Brandt RM, Putavet DA, Klein JD, Derks KW, Bourgeois BR, Stryeck S, Rijksen Y, van Willigenburg H, Feijtel DA (2017) Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 169(132–147):e116
Hickson LJ, Prata LGL, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, Herrmann SM, Jensen MD, Jia Q, Jordan KL (2019) Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine 47:446–456
Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM (2017) Targeting cellular senescence prevents age-related bone loss in mice. Nat Med 23:1072–1079
Borghesan M, Hoogaars W, Varela-Eirin M, Talma N, Demaria M (2020) A senescence-centric view of aging: implications for longevity and disease. Trends Cell Biol 30:777–791
Funding
This work was supported by R01 EY024349 (ARD) and UH3 EY031809 (ARD): Core Grant for Vision Research EY01792 all from NEI/NIH; Vision Research Program – Congressionally Directed Medical Research Program VR170180 from the Department of Defense, Unrestricted Grant to the Department and Physician-Scientist Award both from Research to Prevent Blindness.
Author information
Authors and Affiliations
Contributions
M. S. and A. D. conceptualized the review topic. K. C. and R. K. conducted the search for literature, extracted relevant points of discussion, updated reference lists, and contributed to writing of the report. M. S., K. C., R. K., and A. D. were involved in drafting, and contributed to writing of the review. All co-authors approved of the review being submitted for publication.
Corresponding author
Ethics declarations
Informed consent
There were no human participants and/or animals involved in the study; therefore, no informed consent process was applicable in this review article.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mohammad Soleimani and Kasra Cheraqpour contributed equally.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soleimani, M., Cheraqpour, K., Koganti, R. et al. Cellular senescence and ophthalmic diseases: narrative review. Graefes Arch Clin Exp Ophthalmol 261, 3067–3082 (2023). https://doi.org/10.1007/s00417-023-06070-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06070-9