Abstract
Purpose
To compare the characteristics and incidence rates of lesion reactivation after anti-vascular endothelial growth factor (VEGF) treatment in type 3 macular neovascularization (MNV) with and without subretinal fluid (SRF) at baseline.
Methods
This retrospective study included 95 patients diagnosed with type 3 MNV. After the initial loading injections, re-treatment was performed when lesion reactivation occurred defined as the re-accumulation of subretinal or intraretinal fluid or the new development of a retinal/subretinal hemorrhage. The differences in the baseline characteristics and the incidence rates of lesion reactivation were compared between patients with SRF (SRF group, n = 42) and those without SRF (non-SRF group, n = 53).
Results
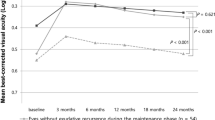
At diagnosis, the mean visual acuity was worse (0.68 ± 0.41 vs 0.50 ± 0.36; P = 0.032), mean central retinal thickness was greater (515.4 ± 145.9 μm vs 383.8 ± 105.5 μm; P < 0.001), and the incidence of focal retinal hemorrhages was higher (90.5% vs 66.0%; P = 0.005) in the SRF group than in the non-SRF group. In the SRF group, the first lesion reactivation was noted in 89.7% at a mean of 5.8 ± 4.4 months after the third injection. In the non-SRF group, the first lesion reactivation was noted in 70.6% at a mean of 6.1 ± 3.8 months. There was a significant difference in lesion reactivation between the two groups (P = 0.019).
Conclusions
The difference in the baseline characteristics and incidence of lesion reactivation between type 3 MNV with and without SRF suggests that the presence of SRF may be indicative of more advanced disease with a high risk of visual deterioration. This result also suggests the need for more active treatment to preserve vision in patients with SRF.



Similar content being viewed by others
References
Chaudhary V, Matonti F, Zarranz-Ventura J, Stewart MW (2022) Impact of fluid compartments on functional outcomes for patients with neovascular age-related macular degeneration: a systematic literature review. Retina 42:589–606
Sacconi R, Forte P, Tombolini B, Grosso D, Fantaguzzi F, Pina A, Querques L, Bandello F, Querques G (2022) OCT predictors of 3-year visual outcome for type 3 macular neovascularization. Ophthalmol Retina 6:586–594
Kim JH, Kim JW, Kim CG (2022) Difference between the incidence of retinal fluid subtypes and their association with visual outcomes according to the types of macular neovascularization in a Korean population. J Ocul Pharmacol Ther 38:261–268
Sharma A, Cheung CMG, Arias-Barquet L, Ozdek S, Parachuri N, Kumar N, Hilely A, Zur D, Loewenstein A, Vella G, Bandello F, Querques G (2022) Fluid-based visual prognostication in type 3 macular neovascularization-flip-3 study. Retina 42:107–113
Freund KB, Ho IV, Barbazetto IA, Koizumi H, Laud K, Ferrara D, Matsumoto Y, Sorenson JA, Yannuzzi L (2008) Type 3 neovascularization: the expanded spectrum of retinal angiomatous proliferation. Retina 28:201–211
Yannuzzi LA, Negrão S, Iida T, Carvalho C, Rodriguez-Coleman H, Slakter J, Freund KB, Sorenson J, Orlock D, Borodoker N (2012) Retinal angiomatous proliferation in age–related macular degeneration 2001. Retina 32(Suppl 1):416–434
Su D, Lin S, Phasukkijwatana N, Chen X, Tan A, Freund KB, Sarraf D (2016) An updated staging system of type 3 neovascularization using spectral domain optical coherence tomography. Retina 36(Suppl 1):S40–S49
Sharma A, Parachuri N, Kumar N, Bandello F, Kuppermann BD, Loewenstein A, Regillo C, Chakravarthy U (2021) Fluid-based prognostication in n-AMD: type 3 macular neovascularisation needs an analysis in isolation. Br J Ophthalmol 105:297–298
Nagiel A, Sarraf D, Sadda SR, Spaide RF, Jung JJ, Bhavsar KV, Ameri H, Querques G, Freund KB (2015) Type 3 neovascularization: evolution, association with pigment epithelial detachment, and treatment response as revealed by spectral domain optical coherence tomography. Retina 35:638–647
Kim JH, Chang YS, Kim JW, Kim CG, Lee DW (2019) Long-term incidence and timing of reactivation in patients with type 3 neovascularization after initial treatment. Graefes Arch Clin Exp Ophthalmol 257:1183–1189
Kim JH, Chang YS, Kim JW, Kim CG, Lee DW (2019) Age-related differences in the prevalence of subtypes of neovascular age-related macular degeneration in the first diagnosed eye. Graefes Arch Clin Exp Ophthalmol 257:891–898
Tsai ASH, Cheung N, Gan ATL, Jaffe GJ, Sivaprasad S, Wong TY, Cheung CMG (2017) Retinal angiomatous proliferation. Surv Ophthalmol 62:462–492
Kim JH, Chang YS, Kim JW, Kim CG, Lee DW, Kim HS (2019) Long-term visual changes in initially stronger fellow eyes in patients with unilateral type 3 neovascularization. Retina 39:1672–1681
Invernizzi A, Teo K, Nguyen V, Daniell M, Squirrell D, Barthelmes D, Gillies MC (2019) Type 3 neovascularisation (retinal angiomatous proliferation) treated with antivascular endothelial growth factor: real-world outcomes at 24 months. Br J Ophthalmol 103:1337–1341
Grunwald JE, Pistilli M, Daniel E, Ying GS, Pan W, Jaffe GJ, Toth CA, Hagstrom SA, Maguire MG, Martin DF (2017) Incidence and growth of geographic atrophy during 5 years of comparison of age-related macular degeneration treatments trials. Ophthalmol 124:97–104
Kim JH, Kim JW, Kim CG, Lee DW (2020) Long-term treatment outcomes in type 3 neovascularization: focus on the difference in outcomes between geographic atrophy and fibrotic scarring. J Clin Mid 9:1145
Freund KB, Korobelnik JF, Devenyi R, Framme C, Galic J, Herbert E, Hoerauf H, Lanzetta P, Michels S, Mitchell P, Mones J, Regillo C, Tadayoni R, Talks J, Wolf S (2015) Treat-and-extend regimens with anti-vegf agents in retinal diseases: a literature review and consensus recommendations. Retina 35:1489–1506
Haj Najeeb B, Deak G, Schmidt-Erfurth U, Gerendas BS (2020) The RAP STUDY, REPORT TWO: the regional distribution of macular neovascularization type 3, a novel insight into its etiology. Retina 40:2255–2262
Kim JH, Chang YS, Kim JW, Kim CG, Lee DW (2020) Characteristics of type 3 neovascularization lesions: focus on the incidence of multifocal lesions and the distribution of lesion location. Retina 40:1124–1131
Rosenberg D, Deonarain DM, Gould J, Sothivannan A, Phillips MR, Sarohia GS, Sivaprasad S, Wykoff CC, Cheung CMG, Sarraf D, Bakri SJ, Chaudhary V (2022) Efficacy, safety, and treatment burden of treat-and-extend versus alternative anti-VEGF regimens for nAMD: a systematic review and meta-analysis. Eye (Lond). https://doi.org/10.1038/s41433-022-02020-7
Arias L, Cervera E, Vilimelis JC, Escobar JJ, Escobar AG, Zapata M (2020) Efficacy and safety of a treat-and-extend regimen with aflibercept in treatment-naive patients with type 3 neovascularization: a 52-week, single-arm, multicenter trial. Retina 40:1234–1244
Shin JY, Yu HG (2014) Optical coherence tomography-based ranibizumab monotherapy for retinal angiomatous proliferation in Korean patients. Retina 34:2359–2366
Engelbert M, Zweifel SA, Freund KB (2009) “Treat and extend” dosing of intravitreal antivascular endothelial growth factor therapy for type 3 neovascularization/retinal angiomatous proliferation. Retina 29:1424–1431
McBain VA, Kumari R, Townend J, Lois N (2011) Geographic atrophy in retinal angiomatous proliferation. Retina 31:1043–1052
Sadda SR, Guymer R, Monés JM, Tufail A, Jaffe GJ (2020) Anti-vascular endothelial growth factor use and atrophy in neovascular age-related macular degeneration: systematic literature review and expert opinion. Ophthalmol 127:648–659
Kim JH, Lee TG, Kim JW, Kim CG, Cho SW, Han JI (2014) Small retinal haemorrhages accompanied by macular soft drusen: prevalence, and funduscopic and angiographic characteristics. Br J Ophthalmol 98:1066–1072
Spaide RF (2019) New proposal for the pathophysiology of type 3 neovascularization as based on multimodal imaging findings. Retina 39:1451–1464
Funding
Kim’s Eye Hospital Research Center (Seoul, South Korea) provided funding for English editing support. The sponsor had no role in the design or conduct of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Institutional Review Board of Kim’s Eye Hospital (Seoul, South Korea) and conducted in accordance with the tenets of the Declaration of Helsinki.
Informed consent
Informed consent was not obtained as no participant information was identified in this study.
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, J.H., Kim, J.W. & Kim, C.G. Difference in characteristics and lesion reactivation between type 3 macular neovascularization with and without subretinal fluid at baseline. Graefes Arch Clin Exp Ophthalmol 261, 401–408 (2023). https://doi.org/10.1007/s00417-022-05833-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-022-05833-0