Abstract
Purpose
To compare iris affectation in cytomegalovirus anterior uveitis (CMVAU), rubella virus-associated uveitis (RVU), and healthy contralateral eyes, using swept-source anterior segment optical coherence tomography (SS-AS-OCT).
Materials and methods
A comparative, transversal, retrospective study examining 60 eyes from 30 patients—18 eyes (17 patients) with CMVAU, 14 eyes (13 patients) with RVU, and 28 healthy eyes—was performed. Six-millimeter cross-sectional SS-AS-OCT B-scans were obtained in each iris quadrant. Images were exported to ImageJ®. Qualitative and quantitative analyses were done: stromal thickness (ST), smooth index (SI), and optical density (OD) of pigment epithelium. Comparisons between measurements and clinical-demographic parameters were performed using SPSS®.
Results
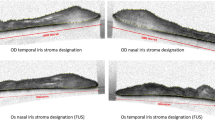
Qualitative analysis showed extensive damage in iris stroma but was unable to differentiate between both uveitis. RVU eyes had a lower mean ST (315.72 μm ± 42.4) compared to those with CMVAU (348.6 μm ± 46) (p = 0.047) and a lower ST in the upper (338.9 μm ± 38.52)/(386.25 μm ± 47.2) (p = 0.005) and temporal (281.5 μm ± 57.3)/(326.43 μm ± 62.3) (p = 0.016) quadrants. Mean (0.94 ± 0.02)/(0.9 ± 0.03) (p = 0.001), temporal (0.94 ± 0.02)/(0.89 ± 0.03) (p < 0.001), and nasal SI (0.094 ± 0.02)/(0.9 ± 0.04) (p = 0.005) were higher in RVU. OD was similar in both uveitis. In healthy eyes, mean ST (376.8 μm ± 39.7) was higher and mean SI was lower (0.87 ± 0.04) than in RVU (p < 0.001) and CMVAU eyes (p = 0.032). Mean OD was higher in healthy eyes (911 ± 130) than in CMVAU eyes (800 ± 200) (p = 0.037).
Conclusions
The quantitative analysis of the SS-AS-OCT iris images allows for the differentiation between healthy eyes and those with CMVAU and RVU, as well as between both uveitis.


Similar content being viewed by others
References
Chan NS, Chee SP, Caspers L, Bodaghi B (2018) Clinical features of CMV-associated anterior uveitis. Ocul Immunol Inflamm 26(1):107–115. https://doi.org/10.1080/09273948.2017.1394471
Wensing B, Relvas LM, Caspers LE, Valentincic NV, Stunf S, de Groot-Mijnes JD, Rothova A (2011) Comparison of rubella virus- and herpes virus-associated anterior uveitis: clinical manifestations and visual prognosis. Ophthalmology 118(10):1905–1910. https://doi.org/10.1016/j.ophtha.2011.03.033
De Groot-Mijnes JD, Rothova A, Van Loon AM, Schuller M, Ten Dam-Van Loon NH, De Boer JH, Schuurman R, Weersink AJ (2006) Polymerase chain reaction and Goldmann-Witmer coefficient analysis are complimentary for the diagnosis of infectious uveitis. Am J Ophthalmol 141(2):313–318. https://doi.org/10.1016/j.ajo.2005.09.017
Chan NS, Chee SP (2019) Demystifying viral anterior uveitis: a review. Clin Experiment Ophthalmol 47(3):320–333. https://doi.org/10.1111/ceo.13417
Kishi S (2016) Impact of swept source optical coherence tomography on ophthalmology. Taiwan J Ophthalmol 6(2):58–68. https://doi.org/10.1016/j.tjo.2015.09.002
Agrawal R, Jain M, Khan R, Jaisankar D, Xin W, Ding J, Testi I, Raman R, Biswas J (2021) Choroidal structural changes in sympathetic ophthalmia on swept-source optical coherence tomography. Ocul Immunol Inflamm 29(3):537–542. https://doi.org/10.1080/09273948.2019.1685110
Agrawal R, Salman M, Tan KA, Karampelas M, Sim DA, Keane PA, Pavesio C (2016) Choroidal vascularity index (CVI)–a novel optical coherence tomography parameter for monitoring patients with panuveitis? PLoS One 11(1):e0146344. https://doi.org/10.1371/journal.pone.0146344
Escribano López P, González-Guijarro JJ (2019) Bilateral acute depigmentation of the iris: findings in anterior segment swept-source optical coherence tomography. Ocul Immunol Inflamm 27(8):1288–1292. https://doi.org/10.1080/09273948.2019.1582784
Jiang J, Yang L, Li H, Huang L, Li N (2019) Evaluation of the iris thickness changes for the Chinese families with GPR143 gene mutations. Exp Eye Res 189:107819. https://doi.org/10.1016/j.exer.2019.107819
Basarir B, Altan C, Pinarci EY, Celik U, Satana B, Demirok A (2013) Analysis of iris structure and iridocorneal angle parameters with anterior segment optical coherence tomography in Fuchs’ uveitis syndrome. Int Ophthalmol 33(3):245–250. https://doi.org/10.1007/s10792-012-9680-8
Invernizzi A, Cigada M, Savoldi L, Cavuto S, Fontana L, Cimino L (2014) In vivo analysis of the iris thickness by spectral domain optical coherence tomography. Br J Ophthalmol 98(9):1245–1249. https://doi.org/10.1136/bjophthalmol-2013-304481
Ozer MD, Kebapci F, Batur M, Seven E, Tekin S (2019) In vivo analysis and comparison of anterior segment structures of both eyes in unilateral Fuchs’ uveitis syndrome. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 257(7), 1489–1498. https://doi.org/10.1007/s00417-019-04351-w
Zarei M, Ebrahimiadib N, Riazi-Esfahani H (2019) Using anterior segment optical coherence tomography to compare the smoothness of anterior iris surface between two eyes in unilateral Fuchs’ uveitis syndrome. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 257(12):2799–2800. https://doi.org/10.1007/s00417-019-04477-x
Zarei M, KhaliliPour E, Ebrahimiadib N, Riazi-Esfahani H (2020) Quantitative analysis of the iris surface smoothness by anterior segment optical coherence tomography in Fuchs uveitis. Ocular immunology and inflammation, 1–6. Advance online publication. https://doi.org/10.1080/09273948.2020.1823424
Martín Ramírez A, Cardeñoso Domingo L, González Guijarro JJ (2019) PCR multiplex for CMV detection in patients with anterior uveitis. Ocul Immunol Inflamm 27(2):197–202. https://doi.org/10.1080/09273948.2018.1438633
Quentin CD, Reiber H (2004) Fuchs heterochromic cyclitis: rubella virus antibodies and genome in aqueous humor. Am J Ophthalmol 138(1):46–54. https://doi.org/10.1016/j.ajo.2004.02.055
Groen-Hakan F, van de Laar S, van der Eijk-Baltissen AA, Ten Dam-van Loon N, de Boer J, Rothova A (2019) Clinical Manifestations, prognosis, and vaccination status of patients with rubella virus-associated uveitis. Am J Ophthalmol 202:37–46. https://doi.org/10.1016/j.ajo.2019.02.002
De Simone L, Belloni L, Aldigeri R, Zerbini A, Mastrofilippo V, Sangiovanni A, Parmeggiani M, Fontana L, Cimino L (2019) Aqueous tap and rapid diagnosis of cytomegalovirus anterior uveitis: the Reggio Emilia experience. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 257(1):181–186. https://doi.org/10.1007/s00417-018-4180-9
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675. https://doi.org/10.1038/nmeth.2089
Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group (2005) Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 140(3):509–516. https://doi.org/10.1016/j.ajo.2005.03.057
Babu K, Konana VK, Ganesh SK, Patnaik G, Chan N, Chee SP, Sobolewska B, Zierhut M (2020) Viral anterior uveitis. Indian J Ophthalmol 68(9):1764–1773. https://doi.org/10.4103/ijo.IJO_928_20
Marsh RJ, Easty DL, Jones BR (1974) Iritis and iris atrophy in Herpes zoster ophthalmicus. Am J Ophthalmol 78(2):255–261. https://doi.org/10.1016/0002-9394(74)90086-5
Sungur GK, Hazirolan D, Yalvac IS, Ozer PA, Aslan BS, Duman S (2010) Incidence and prognosis of ocular hypertension secondary to viral uveitis. Int Ophthalmol 30(2):191–194. https://doi.org/10.1007/s10792-009-9305-z
Baldwin J, Park PJ, Zanotti B, Maus E, Volin MV, Shukla D, Tiwari V (2013) Susceptibility of human iris stromal cells to herpes simplex virus 1 entry. J Virol 87(7):4091–4096. https://doi.org/10.1128/JVI.03235-12
De Groot-Mijnes J, Chan A, Chee SP, Verjans G (2018) Immunopathology of virus-induced anterior uveitis. Ocul Immunol Inflamm 26(3):338–346. https://doi.org/10.1080/09273948.2018.1439069
Accorinti M, Gilardi M, Pirraglia MP, Amorelli GM, Nardella C, Abicca I, Pesci FR (2014) Cytomegalovirus anterior uveitis: long-term follow-up of immunocompetent patients. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 252(11):1817–1824. https://doi.org/10.1007/s00417-014-2782-4
Hiller R, Sperduto RD, Krueger DE (1982) Race, iris pigmentation, and intraocular pressure. Am J Epidemiol 115(5):674–683. https://doi.org/10.1093/oxfordjournals.aje.a113350
Stjernschantz JW, Albert DM, Hu DN, Drago F, Wistrand PJ (2002) Mechanism and clinical significance of prostaglandin-induced iris pigmentation. Surv Ophthalmol 47(Suppl 1):S162–S175. https://doi.org/10.1016/s0039-6257(02)00292-8
Acknowledgements
The authors would like to thank Rodrigo Lisazo, Lorena Vega, Santiago Clement, and the Immunology and Microbiology Services of the Hospital Universitario de la Princesa and Hospital Universitario Puerta de Hierro for their kind contribution to this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Patricia Escribano and J. Jacobo González. The first draft of the manuscript was written by Patricia Escribano and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval was obtained from the Comité de Ética de La Investigación con Medicamentos, Hospital Universitario de la Princesa (reg. number: 4717). All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent
The institutional review board ruled that informed consent was not required for this study.
Conflict of interest
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. None of the authors have any financial interest in any material mentioned in this article. The authors have no conflicts of interest. All authors attest that they meet the current ICMJE criteria for authorship.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Escribano Lopez, P., Gonzalez Guijarro, J.J. Iridian anterior segment OCT in rubella uveitis syndrome and cytomegalovirus anterior uveitis: a comparative study. Graefes Arch Clin Exp Ophthalmol 260, 3647–3655 (2022). https://doi.org/10.1007/s00417-022-05733-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-022-05733-3