Abstract
Purpose
The purpose of this study is to report the 24-month outcomes of a pro re nata (PRN) compared with a treat and extend (T&E) regimen in patients previously treated for neovascular age-related macular degeneration (nAMD).
Methods
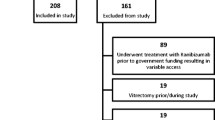
This was a 2-year prospective, single-center study. Previously treated patients for nAMD were randomized into two regimen groups: T&E and PRN groups. Main outcome measured was change in best corrected visual acuity (BCVA) from baseline to month 24. Secondary outcomes encompassed anatomical features such as central retinal thickness (CRT), number of intravitreal injections (IVI), and visits required.
Results
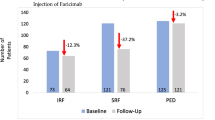
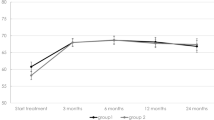
A total of 124 eyes received the T&E (n = 61) or PRN (n = 63) regimen. At month 24, the mean BCVA change was −4.4 early treatment diabetic retinopathy study (ETDRS) letters (T&E) and −3.4 ETDRS letters (PRN), with a difference of +1.1 ETDRS letters (95% CI [−2.25]; p = 0.006). The mean change in CRT was −10.6 µm (T&E) and −7.9 µm (PRN), with a difference of +2.6 µm (95% CI [+19.2]; p = 0.004). The T&E group had received a mean of +4.6 more injections (95% CI [−7.06; −2.12]; p < 0.001) at month 24.
Conclusion
There was statistically proven non-inferiority between the PRN and T&E regimens in terms of visual and anatomical outcomes at 24 months, with significantly more IVI administered in the T&E regimen.





Similar content being viewed by others
References
Pascolini D, Mariotti SP (2012) Global estimates of visual impairment: 2010. Br J Ophthalmol 96:614–618. https://doi.org/10.1136/bjophthalmol-2011-300539
Rosenfeld PJ, Brown DM, Heier JS et al (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355:1419–1431. https://doi.org/10.1056/NEJMoa054481
Brown DM, Kaiser PK, Michels M et al (2006) Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 355:1432–1444. https://doi.org/10.1056/NEJMoa062655
Holz FG, Tadayoni R, Beatty S et al (2015) Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol 99:220–226. https://doi.org/10.1136/bjophthalmol-2014-305327
Holz FG, Amoaku W, Donate J et al (2011) Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology 118:663–671. https://doi.org/10.1016/j.ophtha.2010.12.019
Lalwani GA, Rosenfeld PJ, Fung AE et al (2009) A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol 148:43-58.e1. https://doi.org/10.1016/j.ajo.2009.01.024
Spaide R (2007) Ranibizumab according to need: a treatment for age-related macular degeneration. Am J Ophthalmol 143:679–680. https://doi.org/10.1016/j.ajo.2007.02.024
Engelbert M, Zweifel SA, Freund KB (2010) Long-term follow-up for type 1 (subretinal pigment epithelium) neovascularization using a modified “treat and extend” dosing regimen of intravitreal antivascular endothelial growth factor therapy. Retina (Philadelphia, Pa) 30:1368–1375. https://doi.org/10.1097/IAE.0b013e3181d50cbf
Chin-Yee D, Eck T, Fowler S et al (2016) A systematic review of as needed versus treat and extend ranibizumab or bevacizumab treatment regimens for neovascular age-related macular degeneration. Br J Ophthalmol 100:914–917. https://doi.org/10.1136/bjophthalmol-2015-306987
Shah GK, Stone TW (eds) (2017) Global trends in retina survey: Chicago, IL. American Society of Retina Specialists.
Gillies MC, Walton RJ, Arnold JJ et al (2014) Comparison of outcomes from a phase 3 study of age-related macular degeneration with a matched, observational cohort. Ophthalmology 121:676–681. https://doi.org/10.1016/j.ophtha.2013.09.050
Guymer RH, Markey CM, McAllister IL et al (2019) Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology 126:723–734. https://doi.org/10.1016/j.ophtha.2018.11.025
Heier JS, Brown DM, Chong V et al (2012) Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 119:2537–2548. https://doi.org/10.1016/j.ophtha.2012.09.006
Grunwald JE, Daniel E, Huang J et al (2014) Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 121:150–161. https://doi.org/10.1016/j.ophtha.2013.08.015
Mathalone N, Arodi-Golan A, Sar S et al (2012) Sustained elevation of intraocular pressure after intravitreal injections of bevacizumab in eyes with neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 250:1435–1440. https://doi.org/10.1007/s00417-012-1981-0
Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Martin DF, Maguire MG et al (2012) Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 119:1388–1398. https://doi.org/10.1016/j.ophtha.2012.03.053
Cohen SY, Mimoun G, Oubraham H et al (2013) Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the LUMIERE study. Retina (Philadelphia, Pa) 33:474–481. https://doi.org/10.1097/IAE.0b013e31827b6324
Kim LN, Mehta H, Barthelmes D et al (2016) Metaanalysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina (Philadelphia, Pa) 36:1418–1431. https://doi.org/10.1097/IAE.0000000000001142
Monés J, Singh RP, Bandello F et al (2020) Undertreatment of neovascular age-related macular degeneration after 10 years of anti-vascular endothelial growth factor therapy in the real world: the need for a change of mindset. Ophthalmologica 243:1–8. https://doi.org/10.1159/000502747
Silva R, Berta A, Larsen M et al (2018) Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND study. Ophthalmology 125:57–65. https://doi.org/10.1016/j.ophtha.2017.07.014
Wykoff CC, Croft DE, Brown DM et al (2015) Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Ophthalmology 122:2514–2522. https://doi.org/10.1016/j.ophtha.2015.08.009
Ohji M, Takahashi K, Okada AA et al (2020) Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR. Adv Ther 37:1173–1187. https://doi.org/10.1007/s12325-020-01236-x
Berg K, Pedersen TR, Sandvik L, Bragadóttir R (2015) Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology 122:146–152. https://doi.org/10.1016/j.ophtha.2014.07.041
Barthelmes D, Nguyen V, Daien V et al (2018) Two year outcomes of “treat and extend” intravitreal therapy using aflibercept preferentially for neovascular age-related macular degeneration. Retina (Philadelphia, Pa) 38:20–28. https://doi.org/10.1097/IAE.0000000000001496
Kim JH (2020) Results of switching from pro re nata to treat-and-extend regimen in treatment of patients with type 3 neovascularization. Seminars in Ophthalmology 35:33–40. https://doi.org/10.1080/08820538.2019.1701045
Kvannli L, Krohn J (2017) Switching from pro re nata to treat-and-extend regimen improves visual acuity in patients with neovascular age-related macular degeneration. Acta Ophthalmol 95:678–682. https://doi.org/10.1111/aos.13356
Johnston RL, Carius H-J, Skelly A et al (2017) A Retrospective study of ranibizumab treatment regimens for neovascular age-related macular degeneration (nAMD) in Australia and the United Kingdom. Adv Ther 34:703–712. https://doi.org/10.1007/s12325-017-0483-1
Brown DM, Tuomi L, Shapiro H, Pier Study Group (2013) Anatomical measures as predictors of visual outcomes in ranibizumab-treated eyes with neovascular age-related macular degeneration. Retina (Philadelphia, Pa) 33:23–34. https://doi.org/10.1097/IAE.0b013e318263cedf
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The ethical committee of the Besancon University Hospital (Bourgogne & Franche-Comté University) approved the study.
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Summary statement
Treat and extend regimen is a popular treatment strategy for neovascular age-related macular degeneration. However, data on the efficacy of a regimen switch are limited in patients already treated according to a pro re nata regimen. At 24 months, the non-inferiority of the pro re nata regimen was demonstrated in previously treated patients.
Rights and permissions
About this article
Cite this article
Faudi, E., Gauthier, AS., Delbosc, B. et al. To investigate treat and extend versus pro re nata regimen in neovascular age-related macular degeneration: results from the IDEM study. Graefes Arch Clin Exp Ophthalmol 260, 2149–2156 (2022). https://doi.org/10.1007/s00417-021-05543-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05543-z