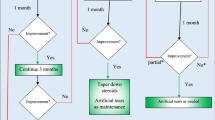
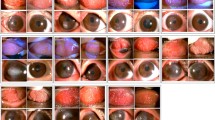
Abstract
Purpose
To compare the effects of ciclosporine A (2%) eye drop and tacrolimus (0.03%) eye ointment on children with vernal keratoconjunctivitis (VKC) who were not responding to corticosteroid eye drops.
Methods
A prospective comparative study was carried out on children who were diagnosed with refractory VKC at the ophthalmology clinic in Benha University, Delta area, Egypt, during the period from October 2019 to February 2020.
Results
Fifty-nine patients completed this study. Regarding the individual symptom score, redness, burning, photophobia, and foreign body sensation were significantly reduced in the tacrolimus group compared to those in the ciclosporine A group during the 1st week (p < 0.05). Moreover, the tacrolimus group showed a statistically significant reduction in burning and foreign body sensation at the 4th week (both p = 0.032), and in redness and burning sensation at the 12th week compared to those in the ciclosporine A group (p = 0.005 and 0.048, respectively). The tacrolimus group showed significantly lower mean scores for tarsal conjunctival papillary hypertrophy at the 1st week and 12th week (p = 0.037 and 0.046, respectively), and for punctate erosion and cobblestone papillae at the 1st week (p = 0.029 and 0.037, respectively) than the ciclosporine group. Failure of treatment was observed in 6 patients (19.35%) in the ciclosporine A group and in 5 patients (17.85%) in the tacrolimus group. No serious side effects were detected in any group.
Conclusion
A higher reduction in inflammatory symptoms and signs as well as compliance with tacrolimus 0.03% eye ointment than with ciclosporine A 2% eye drops was observed. Moreover, long-term medication for refractory cases is needed to control inflammation. Overall, our finding suggested that ciclosporine A eye drops and tacrolimus eye ointment could be considered as corticosteroid-sparing drugs in the management of children with refractory VKC.



Similar content being viewed by others
Data availability
The data of patients used to support the findings of this study are restricted by the Benha University Hospital Research Ethics Board. Data are available for researchers who meet the criteria for access to confidential data upon request from Assistant Professor Mohamed Amin Heikal, Department of Ophthalmology, Benha University, Egypt. E-mail: mohammedheikel@gmail.com.
References
Leonardi A, Motterle L, Bortolotti M (2008) Allergy and the eye. Clin Exp Immunol;153 Suppl 1:17–21.
Kiliç A, Gürler B (2006) Topical 2% cyclosporine A in preservative-free artificial tears for the treatment of vernal keratoconjunctivitis. Can J Ophthalmol 41(6):693–698
Zepeda-Ortega B, Rosas-vargas MA, Ito Tsuchiya FM (2007) Conjuntivitis alérgica en la infancia. Rev Alerg Mex 54:41–53
Bonini S, Coassin M, Aronni S, Lambiase A (2004) Vernal keratoconjunctivitis. Eye (Lond) 18(4):345–351
Kumar S (2009) Vernal keratoconjunctivitis: a major review. Acta Ophthalmol 87(2):133–147
Hingorani M, Lightman S (1995) Therapeutic options in ocular allergic disease. Drugs 50(2):208–221
Spadavecchia L, Fanelli P, Tesse R, Brunetti L, Cardinale F, Bellizzi M, Rizzo G, Procoli U, Bellizzi G, Armenio L (2006) Efficacy of 1.25% and 1% topical cyclosporine in the treatment of severe vernal keratoconjunctivitis in childhood. Pediatr Allergy Immunol;17(7) 527–532.
BenEzra D, Matamoros N, Cohen E (1988) Treatment of severe vernal keratoconjunctivitis with cyclosporine A eyedrops. Transplant Proc 20(2 Suppl 2):644–649
Avunduk A, M, Avunduk M, C, Erdöl H, Kapicioglu Z, Akyol N, (2001) Cyclosporine effects on clinical findings and impression cytology specimens in severe vernal keratoconjunctivitis. Ophthalmologica 215:290–293
Ryu EH, Kim JM, Laddha PM, Chung ES, Chung TY(2012) Therapeutic effect of 0.03% tacrolimus ointment for ocular graft versus host disease and vernal keratoconjunctivitis. Korean J Ophthalmol;26(4):241–7.
Chatterjee S, Agrawal D (2016) Tacrolimus in corticosteroid-refractory vernal keratoconjunctivitis. Cornea 35(11):1444–1448
Akpek EK, Dart JK, Watson S, Christen W, Dursun D, Yoo S, O'Brien TP, Schein OD, Gottsch JD (2004) A randomized trial of topical cyclosporin 0.05% in topical steroid-resistant atopic keratoconjunctivitis. Ophthalmology;111(3):476–82.
Chast F, Lemare F, Legeais JM, Batista R, Bardin C, Renard G (2004) Préparation d’un collyre de ciclosporine à 2% [Cyclosporine 2% eye drops preparation]. J Fr Ophtalmol 27(6 Pt 1):567–576
Fiscella RG, Le H, Lam TT, Labib S (1996) Stability of cyclosporine 1% in artificial tears. J Ocul Pharmacol Ther 12(1):1–4
Tacliment ointment 0.03% w/w (2019) Aurolab india company: www.aurolab.com/tacliment_cp.asp.pdf. Accessed July 2021.
Secchi AG, Tognon MS, Leonardi A (1990) Topical use of cyclosporine in the treatment of vernal keratoconjunctivitis. Am J Ophthalmol; 15;110(6):641–5.
Bleik JH, Tabbara KF (1991) Topical cyclosporine in vernal keratoconjunctivitis. Ophthalmology 98(11):1679–1684
Pucci N, Novembre E, Cianferoni A, Lombardi E, Bernardini R, Caputo R, Campa L, Vierucci A (2002) Efficacy and safety of cyclosporine eyedrops in vernal keratoconjunctivitis. Ann Allergy Asthma Immunol 89(3):298–303
Daniell M, Constantinou M, Vu HT, Taylor HR (2006) Randomised controlled trial of topical ciclosporin A in steroid dependent allergic conjunctivitis. Br J Ophthalmol 90(4):461–464
Tatlipinar S, Akpek EK (2005) Topical ciclosporin in the treatment of ocular surface disorders. Br J Ophthalmol 89(10):1363–1367
De Smedt S, Nkurikiye J, Fonteyne Y, Tuft S, De Bacquer D, Gilbert C, Kestelyn P (2012) Topical ciclosporin in the treatment of vernal keratoconjunctivitis in Rwanda, Central Africa: a prospective, randomised, double-masked, controlled clinical trial. Br J Ophthalmol 96(3):323–328
Yücel OE, Ulus ND (2016) Efficacy and safety of topical cyclosporine A 0.05% in vernal keratoconjunctivitis. Singapore Med J;57 (9):507–10
Labcharoenwongs P, Jirapongsananuruk O, Visitsunthorn N, Kosrirukvongs P, Saengin P, Vichyanond P (2012) A double-masked comparison of 0.1% tacrolimus ointment and 2% cyclosporine eye drops in the treatment of vernal keratoconjunctivitis in children. Asian Pac J Allergy Immunol;30 (3):177–84.
Wan Q, Tang J, Han Y, Wang D, Ye H (2018) Therapeutic effect of 0.1% tacrolimus eye drops in the tarsal form of vernal keratoconjunctivitis. Ophthalmic Res;59 (3):126–134.
Kheirkhah A, Zavareh MK, Farzbod F, Mahbod M, Behrouz MJ (2011) Topical 0.005% tacrolimus eye drop for refractory vernal keratoconjunctivitis. Eye (Lond);25 (7):872–80
Kymionis GD, Kankariya VP, Kontadakis GA (2012) Tacrolimus ointment 0.03% for treatment of refractory childhood phlyctenular keratoconjunctivitis. Cornea;31 (8):950–2.
Ohashi Y, Ebihara N, Fujishima H, Fukushima A, Kumagai N, Nakagawa Y, Namba K, Okamoto S, Shoji J, Takamura E, Hayashi K (2010) A randomized, placebo-controlled clinical trial of tacrolimus ophthalmic suspension 0.1% in severe allergic conjunctivitis. J Ocul Pharmacol Ther. 26 (2) 165–74
Fukushima A, Ohashi Y, Ebihara N, Uchio E, Okamoto S, Kumagai N, Shoji J, Takamura E, Nakagawa Y, Namba K, Fujishima H, Miyazaki D (2014) Therapeutic effects of 0.1% tacrolimus eye drops for refractory allergic ocular diseases with proliferative lesion or corneal involvement. Br J Ophthalmol;98 (8):1023–7
Funding
No funds, grants, or other support was received.
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
No conflicts of interest to declare.
Ethics approval
The Institutional Review Board (IRB) and Research Ethics Committee of Ophthalmic Department, Benha University, approved the study)5/2019). The study adhered strictly to the tenets of the Declaration of Helsinki (2013 revision).
Consent to participate
All subjects (patients and their parents) provided informed written consent for participation.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Heikal, M.A., Soliman, T.T., Abousaif, W.S. et al. A comparative study between ciclosporine A eye drop (2%) and tacrolimus eye ointment (0.03%) in management of children with refractory vernal keratoconjunctivitis. Graefes Arch Clin Exp Ophthalmol 260, 353–361 (2022). https://doi.org/10.1007/s00417-021-05356-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05356-0