Abstract
Purpose
To assess the efficacy of a treat-and-extend strategy with intravitreal ranibizumab for radiation-related macular edema.
Methods
Forty eyes with radiation-induced macular edema and decreased visual acuity were enrolled in the phase IIb, prospective clinical trial and randomized into 3 cohorts: (A) monthly ranibizumab, (B) monthly ranibizumab with targeted retinal photocoagulation (TRP), or (C) as-needed ranibizumab and TRP. In year 2, all subjects entered a treat-and-extend protocol for ranibizumab. The primary outcome measure was mean change in early treatment diabetic retinopathy study (ETDRS) best-corrected visual acuity (BCVA) from baseline.
Results
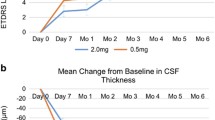
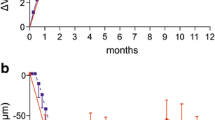
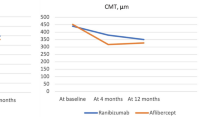
Through year 1, the mean change in ETDRS BCVA was significantly different between the three cohorts (p < 0.001); cohort A saw the largest gain with + 4.0 letters. Significant anatomic improvements were also seen in all cohorts. Comparatively, through year 2, cohorts A, B, and C had a mean change in ETDRS BCVA of − 1.9, − 3.9, and + 1.3 letters, respectively; additionally, no significant differences were found in absolute ETDRS BCVA across time (ANOVA, p = 0.123). Overall, 90% of eyes maintained VA 20/200 or better and 33.3% of subjects gained at least one line of vision. There were no significant differences in mean central macular thickness for any cohort compared to baseline (p = 0.09). The presence of retinal hemorrhage and intraretinal exudates stayed consistent from year 1 to year 2 for all cohorts.
Conclusions
Among eyes with radiation-related macular edema, a treat-and-extend regimen with ranibizumab may not result in as many visual and anatomic improvements as monthly injections. However, treat-and-extend still may prevent serious visual complications compared to historical controls.

Trial registration
ClinicalTrials.gov Identifier: NCT02222610



Similar content being viewed by others
Data availability
The data discussed in the current manuscript will be available on ClinicalTrials.gov under the associated NCT trial code.
Code of availability
N/A
References
Schachat AP (2018) Ryan’s retina, 6th edn. Elsevier, Edinburgh, New York
Finger PT, Chin KJ, Duvall G, Palladium-103 for Choroidal Melanoma Study Group (2009) Palladium-103 ophthalmic plaque radiation therapy for choroidal melanoma: 400 treated patients. Ophthalmology 116(790–796):796.e1. https://doi.org/10.1016/j.ophtha.2008.12.027
Krema H, Somani S, Sahgal A et al (2009) Stereotactic radiotherapy for treatment of juxtapapillary choroidal melanoma: 3-year follow-up. Br J Ophthalmol 93:1172–1176. https://doi.org/10.1136/bjo.2008.153429
Haas A, Pinter O, Papaefthymiou G et al (2002) Incidence of radiation retinopathy after high-dosage single-fraction gamma knife radiosurgery for choroidal melanoma. Ophthalmology 109:909–913. https://doi.org/10.1016/s0161-6420(02)01011-4
Melia BM, Abramson DH, Albert DM et al (2001) Collaborative ocular melanoma study (COMS) randomized trial of I-125 brachytherapy for medium choroidal melanoma. I. Visual acuity after 3 years COMS report no. 16. Ophthalmology 108:348–366. https://doi.org/10.1016/s0161-6420(00)00526-1
Kim IK, Lane AM, Jain P et al (2016) Ranibizumab for the prevention of radiation complications in patients treated with proton beam irradiation for choroidal melanoma. Trans Am Ophthalmol Soc 114:T2
Ramakrishnan S, Anand V, Roy S (2014) Vascular endothelial growth factor signaling in hypoxia and inflammation. J Neuroimmune Pharmacol Off J Soc NeuroImmune Pharmacol 9:142–160. https://doi.org/10.1007/s11481-014-9531-7
Crafts TD, Jensen AR, Blocher-Smith EC, Markel TA (2015) Vascular endothelial growth factor: therapeutic possibilities and challenges for the treatment of ischemia. Cytokine 71:385–393. https://doi.org/10.1016/j.cyto.2014.08.005
Finger PT, Chin KJ (2013) High-dose (2.0 mg) intravitreal ranibizumab for recalcitrant radiation retinopathy. Eur J Ophthalmol 23:850–856. https://doi.org/10.5301/ejo.5000333
Mason JO, Albert MA, Persaud TO, Vail RS (2007) Intravitreal bevacizumab treatment for radiation macular edema after plaque radiotherapy for choroidal melanoma. Retina Phila Pa 27:903–907. https://doi.org/10.1097/IAE.0b013e31806e6042
Finger PT, Mukkamala SK (2011) Intravitreal anti-VEGF bevacizumab (Avastin) for external beam related radiation retinopathy. Eur J Ophthalmol 21:446–451. https://doi.org/10.5301/EJO.2011.6213
Kinyoun JL (2008) Long-term visual acuity results of treated and untreated radiation retinopathy (an AOS thesis). Trans Am Ophthalmol Soc 106:325–335
Finger PT, Chin KJ, Semenova EA (2016) Intravitreal anti-VEGF therapy for macular radiation retinopathy: a 10-year study. Eur J Ophthalmol 26:60–66. https://doi.org/10.5301/ejo.5000670
Schefler AC, Fuller D, Anand R et al (2020) Randomized trial of monthly versus as-needed intravitreal ranibizumab for radiation retinopathy-related macular edema: 1 Year Outcomes. Am J Ophthalmol. https://doi.org/10.1016/j.ajo.2020.03.045
Feder RS, Chuck RS, Dunn SP, et al (2019) Diabetic retinopathy preferred practice pattern
Flaxel CJ, Adelman RA, Bailey ST et al (2020) Age-related macular degeneration Preferred Practice Pattern®. Ophthalmology 127:P1–P65. https://doi.org/10.1016/j.ophtha.2019.09.024
Berg K, Pedersen TR, Sandvik L, Bragadóttir R (2015) Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology 122:146–152. https://doi.org/10.1016/j.ophtha.2014.07.041
Wykoff CC, Ou WC, Brown DM et al (2017) Randomized trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration. Ophthalmol Retina 1:314–321. https://doi.org/10.1016/j.oret.2016.12.004
Payne JF, Wykoff CC, Clark WL et al (2017) Randomized trial of treat and extend ranibizumab with and without navigated laser for diabetic macular edema. Ophthalmology 124:74–81. https://doi.org/10.1016/j.ophtha.2016.09.021
Payne JF, Wykoff CC, Clark WL et al (2019) Randomized trial of treat and extend ranibizumab with and without navigated laser versus monthly dosing for diabetic macular edema: TREX-DME 2-year outcomes. Am J Ophthalmol 202:91–99. https://doi.org/10.1016/j.ajo.2019.02.005
Wykoff CC, Clark WL, Nielsen JS, et al (2018) Optimizing anti-VEGF treatment outcomes for patients with neovascular age-related macular degeneration. J Manag Care Spec Pharm 24:S3–S15. https://doi.org/10.18553/jmcp.2018.24.2-a.s3
Busbee BG, Ho AC, Brown DM et al (2013) Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 120:1046–1056. https://doi.org/10.1016/j.ophtha.2012.10.014
Shields CL, Dalvin LA, Chang M et al (2019) Visual outcome at 4 years following plaque radiotherapy and prophylactic intravitreal bevacizumab (every 4 months for 2 years) for uveal melanoma: comparison with nonrandomized historical control individuals. JAMA Ophthalmol. https://doi.org/10.1001/jamaophthalmol.2019.5132
Acknowledgements
The authors would also like to acknowledge Guangming Lang for providing significant statistical analyses of the data presented in this manuscript. He has been financially compensated for his contributions.
Funding
This work was supported by Genentech, Inc (San Francisco, CA).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethics approval
Institutional Review Board/Ethics Committee approval was prospectively obtained by the Houston Methodist Hospital (Houston, TX, USA).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
All participants of the current study signed informed consent regarding publishing their data and photographs.
Conflict of interest
Dwain Fuller, Rajiv Anand, and Tim Fuller report investigator funding from Genentech Inc. Amy C. Schefler reports investigator funding and consultant fees from Genentech Inc. All other authors have no financial disclosures to report.
Disclaimer
The sponsor or funding organization had no role in the design or conduct of this research and writing of as well as the decision to submit the article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix Additional RRR Study Group Members:
Appendix Additional RRR Study Group Members:
Maria E. Bretana1.
Karen Diugnan2.
1Retina Consultants of Texas, Retina Consultants of America, Houston, TX, USA.
2Texas Retina Associates, Dallas, TX, USA.
Rights and permissions
About this article
Cite this article
Yu, H.J., Fuller, D., Anand, R. et al. Two-year results for ranibizumab for radiation retinopathy (RRR): a randomized, prospective trial. Graefes Arch Clin Exp Ophthalmol 260, 47–54 (2022). https://doi.org/10.1007/s00417-021-05281-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05281-2