Abstract
Purpose
To identify risk factors for fellow eye treatment of diabetic retinopathy with Vascular Endothelial Growth Factor (VEGF) injections during the Diabetic Retinopathy Clinical Research Network (DRCR.Net) Protocol T trial
Methods
In this post-hoc analysis of randomized clinical trial data, Cox regression analysis was performed at 52 and 104 weeks to determine risk factors for treatment in 360 fellow eyes. Survival analysis was performed to determine mean time to treatment based upon medication used.
Results
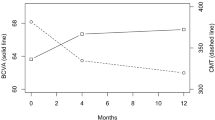
Of 360 fellow eyes, 142 (39.4%) required treatment between weeks 4 and 104. Risk factors predicting a lower likelihood of year 1 treatment included older subject age (Hazard Ratio [HR]=0.98, 95% CI 0.96–0.99; p = 0.02) and higher baseline study eye ETDRS score (HR=0.98, 95% CI 0.97-0.99, p = 0.04). Center-involving DME at baseline in the fellow eye was predictive of a higher treatment need at both 52 (HR=1.89, 95% CI 1.42-2.51, p < 0.0001) and 104 weeks (HR=2.68, 95% CI 1.75-4.11, p < 0.0001). Subjects treated in the study eye with aflibercept (HR=0.574, 95% CI 0.371–0.887, p = 0.013) and ranibizumab (HR=0.58, 95%CI 0.36-0.94, p = 0.03) were less likely to require first year fellow eye injection than subjects treated with bevacizumab although this difference was no longer significant at week 104 (aflibercept HR=0.77, 95% CI 0.52–1.16, p = 0.21; ranibizumab HR=0.66, 95% CI 0.43–1.00, p = 0.05). Mean time to treatment was significantly shorter in the bevacizumab group (bevacizumab 25.83 weeks, aflibercept 38.75 weeks, ranibizumab 34.70 weeks (p=0.012)).
Conclusion
Bilateral treatment with intravitreal anti-VEGF injections was common during the DRCR.net Protocol T. Medication choice may impact the risk of fellow eye treatment.

Similar content being viewed by others
Data availability
The source of the data is the DRCR.net, but the analyses, content and conclusions presented herein are solely the responsibility of the authors and have not been reviewed or approved by the DRCR.net. The contents of this report do not represent the views of the United States Department of Veterans Affairs or the United States Government.
Materials availability
All data is available at https://public.jaeb.org/drcrnet/stdy
Code availability
Not applicable
Abbreviations
- VEGF:
-
Vascular endothelial growth factor
- DRCR.Net:
-
Diabetic retinopathy clinical research network
- ETDRS:
-
Early treatment of diabetic retinopathy study
- DME:
-
Diabetic macular edema
- OCT:
-
Optical coherence tomography
- CMT:
-
Central macular thickness
- HbA1c:
-
Glycosylated hemoglobin
- HTN:
-
Hypertension
- MI:
-
Myocardial infarction
- CAD:
-
Coronary artery disease
- TIA:
-
Transient ischemic attack
- CVA:
-
Cerebrovascular accident
- PRP:
-
Panretinal photocoagulation
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- PDR:
-
Proliferative diabetic retinopathy
- NPDR:
-
Nonproliferative diabetic retinopathy
- WESDR:
-
Wisconsin epidemiologic study of diabetic retinopathy
- DM:
-
Diabetes mellitus
- VA:
-
Visual acuity
- DR:
-
Diabetic retinopathy
References
Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, Arnold-Bush B, Baker CW, Bressler NM, Browning DJ, Elman MJ, Ferris FL, Friedman SM, Melia M, Pieramici DJ, Sun JK, Beck RW (2015) Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema. N Engl J Med 372:1193–1203
Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, Brucker AJ, Ferris FL, Hampton GR, Jhaveri C, Melia M, Beck RW, Diabetic Retinopathy Clinical Research Network (2016) Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology 123:1351–1359
Duker JS, Brown GC, Bosley TM, Colt CA, Reber R (1990) Asymmetric proliferative diabetic retinopathy and carotid artery disease. Ophthalmology 97:869–874
Klein R, Klein BE, Moss SE, Davis MD, Demets DL (1984) The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology 91:1464-1474
Calvo CM, Sridhar J, Shahlaee A, Ho AC (2016) Reduction of Diabetic Macular Edema in the Untreated Fellow Eye Following Intravitreal Injection of Aflibercept. Ophthalmic Surg Lasers Imaging Retina 47:474–476
Peyman M, Peyman A, Lansingh VC, Orandi A, Subrayan V (2019) Intravitreal bevacizumab versus ranibizumab: Effects on the vessels of the fellow non-treated eye. J Curr Ophthalmol 31:55–60
Scartozzi R, Chao J, Walsh A, Eliott D (2009) Bilateral improvement of persistent diffuse diabetic macular oedema after unilateral intravitreal bevacizumab (Avastin) injection. Eye 23:1229
Hanhart J, Tiosano L, Averbukh E, Bnin E, Hemo I, Chowers I (2014) Fellow eye effect of unilateral intravitreal bevacizumab injection in eyes with diabetic macular edema. Eye 28:646–653
Malbin B, Patel H, He Y, Le K, Lin X (2019) Comparative Eye Effect of Unilateral Intravitreal Bevacizumab, Ranibizumab, and Aflibercept for Diabetic Macular Edema. JVRD 3:86–89
Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R, Couvillion S, Nasir MA, Rabena MD, Maiai M, Van Everen S, Le K, Hanley WD (2017) Systemic Pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina 37:1847–1858
Hirano T, Toriyama Y, Iesato Y, Imai A, Murata T (2018) Changes in plasma vascular endothelial growth factor level after intravitreal injections of bevacizumab, aflibercept, or ranibizumab for diabetic macular edema. Retina 38:1801–1808
Jampol LM, Glassman AR, Liu D, Paul AL, Bressler NM, Duh EJ, Quaggin S, Wells JA, Wykoff CC, for the Diabetic Retinopathy Clinical Research Network (2018) Plasma VEGF Concentrations after Intravitreous Anti-VEGF Therapy for Diabetic Macular Edema. Ophthalmology 125:1054–1063
Bhavsar KV, Subramanian ML (2011) Risk factors for progression of subclinical diabetic macular oedema. Br J Ophthalmol 95:671–674
Diabetic Retinopathy Clinical Research Network, Bressler NM, Miller KM, Beck RW, Bressler SB, Glassman AR, Kitchens JW, Melia M, Schlossman DK (2012) Observational study of subclinical diabetic macular edema. Eye 26:833–840
Browning DJ, Fraser CM (2008) The predictive value of patient and eye characteristics on the course of subclinical diabetic macular edema. Am J Ophthalmol 145:149–154
Baker CW, Glassman AR, Beaulieu WT, Antoszyk AN, Browning DJ, Chalam KV, Grover S, Jampol LM, Jhaveri CD, Melia M, Stockdale CR, Martin DF, Sun JK, Retina Network DRCR (2019) Effect of Initial Management With Aflibercept vs Laser Photocoagulation vs Observation on Vision Loss Among Patients With Diabetic Macular Edema Involving the Center of the Macula and Good Visual Acuity: A Randomized Clinical Trial. JAMA 321:1880–1894
Bressler SB, Odia I, Maguire MG, Dhoot DS, Glassman AR, Jampol LM, Marcus DM, Solomon SD, Sun JK, Diabetic Retinopathy Clinical Research Network (2019) Factors Associated With Visual Acuity and Central Subfield Thickness Changes When Treating Diabetic Macular Edema With Anti-Vascular Endothelial Growth Factor Therapy: An Exploratory Analysis of the Protocol T Randomized Clinical Trial. JAMA Ophthalmol 137:382–389
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest
Ethics approval
The Institutional Review Board of Boston University Medical Campus determined that approval was not required for this study.
Consent to participate
No specific consent was required for this post-hoc data analysis
Consent for publication
No specific consent was required for this post-hoc data analysis
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ness, S., Green, M., Loporchio, D. et al. Risk factors for fellow eye treatment in protocol T. Graefes Arch Clin Exp Ophthalmol 259, 2203–2212 (2021). https://doi.org/10.1007/s00417-021-05108-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05108-0