Abstract
Purpose
Common methods of measuring severity of Fuchs endothelial corneal dystrophy (FECD) are limited in objectivity, reliability, or start with a variable baseline that prevents distinguishing healthy from affected eyes. The aim of this study was to describe a method of grading FECD that overcomes these limitations.
Methods
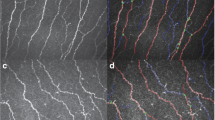
Fifteen patients with Fuchs endothelial corneal dystrophy were included in the study. Guttae were imaged with a slit lamp beam 8 mm tall; the bottom 4 mm half of each image was divided into two equally-sized sections. Guttae were counted by four independent graders blinded to disease severity scores. The peripheral:central guttae ratio was compared to modified Krachmer clinical severity scores. The peripheral:central guttae ratio was compared between mild (severity 0.5–3) versus moderate-to-severe (severity 4–5) disease. Receiver operating characteristics defined optimal ratio cutoffs for mild versus moderate-to-severe disease.
Results
Increased peripheral guttae and peripheral:central guttae ratio correlated with Krachmer severity (p = 0.021 and p = 0.009, respectively). The difference between mild and moderate-to-severe cases for the peripheral:central guttae ratio was significant (p < 0.001). Inter-rater reliability of total guttae count was high (coefficient = 0.82, p < 0.001). A peripheral:central guttae ratio of 0.16 was the ideal cut-off point (area under the curve = 0.79, sensitivity = 0.78, and specificity = 0.80).
Conclusion
In this pilot study, the peripheral:central ratio of guttae correlates with subjective clinical severity of Fuchs dystrophy. It starts at a common baseline, has good inter-rater reliability, does not require dilation, and can be conducted with a smartphone and slit-lamp.


Similar content being viewed by others
Data availability
Study data is maintained by the investigators and is available upon request.
References
Eghrari AO, Riazuddin SA, Gottsch JD (2015) Fuchs corneal dystrophy. Prog Mol Biol Transl Sci 134:79–97
Eye Bank Association of America (2019) Analysis of surgical Use and indications for corneal transplant. 2019 Eye banking Statistical Report. https://restoresight.org/wp-content/uploads/2020/04/2019-EBAA-Stat-Report-FINAL.pdf. Accessed 4 May 2020
Eghrari AO, Gottsch JD (2010) Fuchs’ corneal dystrophy. Expert Rev Ophthalmol 5(2):147–159
Zhang J, Patel DV (2015) The pathophysiology of Fuchs' endothelial dystrophy – A review of molecular and cellular insights. Exp Eye Res 130:97–105
Wilson SE, Bourne WM (1988) Fuchs' dystrophy. Cornea 7(1):2–18
Kopplin LJ, Przepyszny K, Schmotzer B et al (2012) Relationship of Fuchs endothelial corneal dystrophy severity to central corneal thickness. Arch Ophthalmol 130(4):433–439
Feng MT, Kim JT, Ambrósio RJ et al (2012) International values of central Pachymetry in Normal subjects by rotating Scheimpflug camera. Asia Pac J Ophthalmol (Phila) 1:13–18
Repp DJ, Hodge DO, Baratz KH et al (2013) Fuchs' endothelial corneal dystrophy: Subjective grading versus objective grading based on the central-to-peripheral thickness ratio. Ophthalmology 120(4):687–694
McLaren JW, Bachman LA, Kane KM, Patel SV (2014) Objective assessment of the corneal endothelium in Fuchs’ endothelial dystrophy. Invest Ophthalmol Vis Sci 55:1184–1190
Adamis AP, Filatov V, Tripathi BJ et al (1993) Fuchs’ endothelial dystrophy of the cornea. Surv Ophthalmol 38(2):149–168
Krachmer JH, Purcell JJ Jr, Young CW, Bucher KD (1978) Corneal endothelial dystrophy. A study of 64 families. Arch Ophthalmol 96(11):2036–2039
Afshari N, Igo R, Morris N et al (2017) Genome-wide association study identifies three novel loci in Fuchs’ endothelial corneal dystrophy. Nat Commun 8:148–198
Eghrari AO, Mumtaz AA, Garrett B, Rezaei M, Akhavan MS, Riazuddin SA, Gottsch JD (2017) Automated Retroillumination photography analysis for objective assessment of Fuchs’ corneal dystrophy. Cornea 36(1):44–47
Eghrari AO, Garrett BS, Mumtaz AA, Edalati AE, Meadows DN, McGlumphy EJ, Iliff BW, Gottsch JD (2015) Retroillumination photography analysis enhances clinical definition of severe Fuchs’ corneal dystrophy. Cornea. 34(12):1623–1626
Maamari RN, Ausayakhun S, Margolis TP, et al (2014) Novel telemedicine device for diagnosis of corneal abrasions and ulcers in resource-poor settings. JAMA Ophthalmol 132:894–895
He L, Myung D, Pershing S, Chang R (2014) iPhone photography of eye pathology for remote triage. Invest Ophthalmol Vis Sci 55(13):4875
Koo TK, Li MY (2016) A guideline of selecting and reporting Intraclass correlation coefficients for reliability research. J Chiropr Med 15(2):155–163
Moloney G, Petsoglou C, Ball M et al (2017) Descemetorhexis without grafting for Fuchs’ endothelial dystrophy-supplementation with topical Ripasudil. Cornea 36(6):642–648
Yen CH, Wang GQ, Lin TY, Liu CH (2019) Semi-permanent smartphone adapter for microscopes: Design demonstration and workflow testing using a slit-lamp biomicroscope. Taiwan J Ophthalmol 9(2):111–117
Funding
No funding was received for conducting this study. Allen O Eghrari is provided salary support by Research to Prevent Blindness Sybil B. Harrington Special Scholar Award and the Tolsma family. All other authors have no funding sources to disclose.
Author information
Authors and Affiliations
Contributions
Rohan P Bajaj (ORCID 0000–0002–3934-3444): data curation; formal analysis; conceptualization; methodology.
Michael J Fliotsos (ORCID 0000–0001–8429-0831): writing – original draft; writing – review & editing; visualization; project administration.
Tejus Pradeep (ORCID 0000–0002-0506-069X): formal analysis; writing – review & editing.
Allen O Eghrari (ORCID 0000–0003-2798-038X): supervision; conceptualization; methodology; validation; data curation.
Corresponding author
Ethics declarations
Conflict of interest
Allen O Eghrari has an ownership interest in Treyetech and LuckyVision, LLC. All other authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Informed consent / ethical approval
This study was reviewed by the Johns Hopkins University School of Medicine Institutional Review Board (IRB) under study identifier IRB00217273. All study activities adhered to the Declaration of Helsinki. The study was deemed exempt by the IRB under the United States Department of Health and Human Services (DHHS) regulations as a retrospective review of images and data existing in the medical record. The IRB, acting as a HIPAA (Health Insurance Portability and Accountability Act) Privacy Board, reviewed and approved the HIPAA consent waiver via the expedited mechanism as “secondary research” under 45 CFR 46.104(d)(4)(iii) of DHHS regulations.
Sources of support
A. O. Eghrari is supported by the Research to Prevent Blindness Sybil B. Harrington Special Scholar Award and the Tolsma family.
The remaining authors have no funding disclosures to report.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplemental Fig. 1
Receiving operating characteristics curve for peripheral:central ratio of guttae. (JPG 35 kb).
Rights and permissions
About this article
Cite this article
Bajaj, R.P., Fliotsos, M.J., Pradeep, T. et al. Peripheral-to-central ratio of Guttae: validity and reliability of an objective method to characterize severity of Fuchs endothelial corneal dystrophy. Graefes Arch Clin Exp Ophthalmol 259, 685–690 (2021). https://doi.org/10.1007/s00417-020-04985-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-04985-1