Abstract
Purpose
The formation of retinal neovascularization (RNV) is the primary pathological process underlying retinopathy of prematurity (ROP). Previous studies have shown that inflammatory factors are related to the formation of RNV. Tumor necrosis factor-α (TNF-α), as an important factor in the inflammatory response, is involved in the regulation of RNV formation. However, the mechanism through which TNF-α inhibition reduces RNV formation is not fully clarified. Therefore, the purpose of this study was to explore the effect of etanercept, an inhibitor of TNF-α, on RNV, and its possible mechanism.
Methods
In vivo, an oxygen-induced retinopathy (OIR) mouse model was used to determine the effect of etanercept on the formation of RNV by performing immunostaining. The effect of etanercept on tumor necrosis factor receptor-associated factor 2 (TRAF2), pro-angiogenic-related factors, and pro/anti-inflammatory factors in OIR mice was assessed by real-time PCR and Western blotting. In vitro, the effect of etanercept on TNF-α-induced human retinal microvascular endothelial cell tube formation was evaluated by tube formation assays, and the potential mechanism of etanercept was explored by Western blotting.
Results
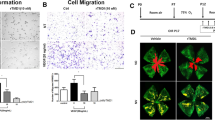
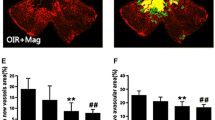
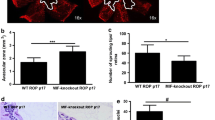
In vivo, etanercept reduced the area of RNV and decreased the expression of TRAF2 in the OIR mouse model. Etanercept also suppressed the expression of several pro-angiogenic factors and regulated the pro/anti-inflammatory factors. In vitro, etanercept reduced endothelial cell tube formation by inhibiting activation of the NF-κB signaling pathway.
Conclusion
Etanercept can regulate pro/anti-inflammatory factors and reduce the expression of pro-angiogenic factors by inhibiting NF-κB phosphorylation, thereby reducing RNV formation.






Similar content being viewed by others
References
Rivera JC, Rabah D, Baraa N et al (2017) Ischemic retinopathies: oxidative stress and inflammation. Oxidative Med Cell Longev 2017:1–16. https://doi.org/10.1155/2017/3940241
Hoppe G, Yoon S, Gopalan B et al (2016) Comparative systems pharmacology of HIF stabilization in the prevention of retinopathy of prematurity. Proc Natl Acad Sci U S A 113(18):2516–2525. https://doi.org/10.1073/pnas.1523005113
Bancalari A, Schade R (2020, 2020) Update in the treatment of retinopathy of prematurity. Am J Perinatol. https://doi.org/10.1055/s-0040-1713181
Morin J, Luu TM, Superstein R et al (2016) Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics 137(4). https://doi.org/10.1542/peds.2015-3218
Cabral T, Mello LGM, Lima LH et al (2017) Retinal and choroidal angiogenesis: a review of new targets. Int J Retina Vitreous 3(1):31. https://doi.org/10.1186/s40942-017-0084-9
Tremblay S, Miloudi K, Chaychi S et al (2013) Systemic inflammation perturbs developmental retinal angiogenesis and neuroretinal function. Invest Ophthalmol Vis Sci 54(13):8125–8139. https://doi.org/10.1167/iovs.13-12496
Nandgaonkar BN, Rotschild T, Yu K et al (1999) Indomethacin improves oxygen-induced retinopathy in the mouse. Pediatr Res 46:184–188. https://doi.org/10.1203/00006450-199908000-00010
Yossuck P, Yan Y, Tadesse M et al (2001) Dexamethasone alters TNF-alpha expression in retinopathy. Mol Genet Metab 72:164–167. https://doi.org/10.1006/mgme.2000.3124
Sharma J, Barr SM, Yixun G et al (2003) Ibuprofen improves oxygen-induced retinopathy in a mouse model. Curr Eye Res 27(5):309–314. https://doi.org/10.1076/ceyr.27.5.309.17222
Wilkinson-Berka Jennifer L, Alousis Nicole S, Kelly Darren J et al (2003) COX-2 inhibition and retinal angiogenesis in a mouse model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 44(3):974–979. https://doi.org/10.1167/iovs.02-0392
Takahashi K, Saishin Y, Mori K et al (2003) Topical nepafenac inhibits ocular neovascularization. Invest Ophthalmol Vis Sci 44:409–415. https://doi.org/10.1167/iovs.02-0346
Yoshida S, Yoshida A, Ishibashi T (2004) Induction of IL-8, MCP-1, and bFGF by TNF-alpha in retinal glial cells: implications for retinal neovascularization during post-ischemic inflammation. Graefes Arch Clin Exp Ophthalmol 242:409–413. https://doi.org/10.1007/s00417-004-0874-2
Kociok N, Radetzky S, Krohne TU et al (2006) Pathological but not physiological retinal neovascularization is altered in TNF-Rp55-receptor–deficient mice. Invest Ophthalmol Vis Sci 47(11). https://doi.org/10.1167/iovs.06-0407
Limb GA, Hollifield RD, Webster L et al (2001) Soluble TNF receptors in vitreoretinal proliferative disease. Invest Ophthalmol Vis Sci 42:1586–1591
Mezu-Ndubuisi OJ, Wanek J, Chau FY et al (2014) Correspondence of retinal thinning and vasculopathy in mice with oxygen-induced retinopathy. Exp Eye Res 122:119–122. https://doi.org/10.1016/j.exer.2014.03.010
Padilla-Mart EM, Romero SC, Bello-Gualtero JM et al (2019) Drug levels and antibodies against TNF-blockers in spondyloarthritis and rheumatoid arthritis are associated with the activity but they do not predict it. Curr Rheumatol Rev 15:329–335
D'Adamio S, Silvaggio D, Massaro A et al (2019) Pharmacotherapeutic management of psoriasis in adolescents and children. Expert Opin Pharmacother 20:1777–1785. https://doi.org/10.1080/14656566.2019.1636032
Bartalena L (2014) Commentary: rituximab, adalimumab, etanercept, tocilizumab--are biologics the future for Graves' orbitopathy? Ophthalmic Plast Reconstr Surg 30:420–423. https://doi.org/10.1097/IOP.0000000000000221
Schwartzman S (2016) Advancements in the management of uveitis. Best Pract Res Clin Rheumatol 30:304–315. https://doi.org/10.1016/j.berh.2016.07.005
Lim H, Lee SH, Lee HT et al (2018) Structural biology of the TNFα antagonists used in the treatment of rheumatoid arthritis. Int J Mol Sci 19:768. https://doi.org/10.3390/ijms19030768
Shen J, Xie B, Dong A et al (2007) Campochiaro PA. In vivo immunostaining demonstrates macrophages associate with growing and regressing vessels. Invest Ophthalmol Vis Sci 48:4335–4341. https://doi.org/10.1167/iovs.07-0113
Zhu Y, Tan W, Demetriades Anna M et al (2016) Interleukin-17A neutralization alleviated ocular neovascularization by promoting M2 and mitigating M1 macrophage polarization. Immunology 147:414–428. https://doi.org/10.1111/imm.12571
Cai Y, Tan W, Shen X et al (2016) Neutralization of IL-23 depresses experimental ocular neovascularization. Exp Eye Res 146:242–251. https://doi.org/10.1016/j.exer.2016.02.008
Yang F, Bai Y, Jiang Y (2015) Effects of Apelin on RAW264.7 cells under both normal and hypoxic conditions. Peptides 69:133–143. https://doi.org/10.1016/j.peptides.2015.04.025
Shirasawa M, Sonoda S, Terasaki H et al (2013) TNF-α disrupts morphologic and functional barrier properties of polarized retinal pigment epithelium. Exp Eye Res 110:59–69. https://doi.org/10.1016/j.exer.2013.02.012
Park SY, Lee SW, Kim HY et al (2015) HMGB1 induces angiogenesis in rheumatoid arthritis via HIF-1α activation. Eur J Immunol 45:1216–1227. https://doi.org/10.1002/eji.201444908
Hassett B, Singh E, Mahgoub E et al (2018) Manufacturing history of etanercept (Enbrel): consistency of product quality through major process revisions. MAbs 10:159–165. https://doi.org/10.1080/19420862.2017.1388483
Fisher BA, Donatien P, Filer A et al (2016) Decrease in articular hypoxia and synovial blood flow at early time points following infliximab and etanercept treatment in rheumatoid arthritis. Clin. Exp Rheumatol 34:1072–1076
Leblond A, Allanore Y, Avouac J (2017) Targeting synovial neoangiogenesis in rheumatoid arthritis. Autoimmun Rev 16:594–601. https://doi.org/10.1016/j.autrev.2017.04.005
Bradley JR (2008) TNF-Mediated inflammatory disease. J Pathol 214(2):149–160. https://doi.org/10.1002/path.2287
Baud V, Karin M (2001) Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol 11:372–377. https://doi.org/10.1016/s0962-8924(01)02064-5
MacEwan DJ (2002) TNF ligands and receptors-a matter of life and death. Br J Pharmacol 135(4):855–875. https://doi.org/10.1038/sj.bjp.0704549
Xie P (2013) TRAF molecules in cell signaling and in human diseases. J Mol Signal 8(1):1–31. https://doi.org/10.1186/1750-2187-8-7
Chung JY, Park YC, Ye H et al (2002) All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J Cell Sci 115(Pt 4):679–688
Yang XD, Sun SC (2015) Targeting signaling factors for degradation, an emerging mechanism for TRAF functions. Immunol Rev 266(1):56–71. https://doi.org/10.1111/imr.12311
Rossi AFT, Contiero JC, Manoel-Caetano FS et al (2019) Up-regulation of tumor necrosis factor-α pathway survival genes and of the receptor TNFR2 in gastric cancer. World J Gastrointest Oncol 11:281–294. https://doi.org/10.4251/wjgo.v11.i4.281
Qiao YQ, Shen J, Gu Y et al (2013) Gene expression of tumor necrosis factor receptor associated-factor (TRAF)-1 and TRAF-2 in inflammatory bowel disease. J Dig Dis 14:244–250. https://doi.org/10.1111/1751-2980.12044
Romano S, D'Arrigo P, Tufano M et al (2019) TRAF2 and FKBP51 as possible markers for identification of suitable melanoma tumors for tumor necrosis factor-α inhibition. Melanoma Res 29:145–150. https://doi.org/10.1097/CMR.0000000000000553
Zhang W, Sun Y, Liu L et al (2017) Prognostic significance of TNFR-associated factor 1 and 2 (TRAF1 and TRAF2) in glioblastoma. Med Sci Monit 23:4506–4512. https://doi.org/10.12659/msm.903397
Sato T, Kusaka S, Hashida N et al (2009) Comprehensive gene-expression profile in murine oxygen-induced retinopathy. Br J Ophthalmol 93(1):96–103. https://doi.org/10.1136/bjo.2008.142646
Brockhaus M, Schoenfeld HJ, Schlaeger EJ et al (1990) Identification of two types of tumor necrosis factor receptors on human cell lines by monoclonal antibodies. Proc Natl Acad Sci U S A 87:3127–3131. https://doi.org/10.1073/pnas.87.8.3127
Bradley JR, Thiru S, Pober JS (1995) Disparate localization of 55-kd and 75-kd tumor necrosis factor receptors in human endothelial cells. Am J Pathol 146:27–32
Sato T, Kusaka S, Shimojo H et al (2009) Simultaneous analyses of vitreous levels of 27 cytokines in eyes with retinopathy of prematurity. Ophthalmology 116:2165–2169. https://doi.org/10.1016/j.ophtha.2009.04.026
Kataoka K, Nishiguchi KM, Kaneko H et al (2011) The roles of vitreal macrophages and circulating leukocytes in retinal neovascularization. Invest Ophthalmol Vis Sci 52:1431–1438. https://doi.org/10.1167/iovs.10-5798
Suganuma M, Okabe S, Marino MW et al (1999) Essential role of tumor necrosis factor alpha (TNF-alpha) in tumor promotion as revealed by TNF-alpha-deficient mice. Cancer Res 59:4516–4518
Zhu XY, Daghini E, Chade AR et al (2008) Disparate effects of simvastatin on angiogenesis during hypoxia and inflammation. Life Sci 83:801–809. https://doi.org/10.1016/j.lfs.2008.09.029
Leibovich SJ, Polverini PJ, Shepard HM et al (1987) Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature 329:630–632. https://doi.org/10.1038/329630a0
Balkwill F (2009) Tumour necrosis factor and cancer. Nat Rev Cancer 9(5):361–371. https://doi.org/10.1016/0955-2235(92)90027-f
Aggarwal BB, Shishodia S, Sandur SK et al (2006) Inflammation and cancer: how hot is the link? Biochem Pharmacol 72(11):1605–1621. https://doi.org/10.1016/j.bcp.2006.06.029
Tang SC, Liao PY, Hung SJ et al (2017) Topical application of glycolic acid suppresses the UVB induced IL-6, IL-8, MCP-1 and COX-2 inflammation by modulating NF-κB signaling pathway in keratinocytes and mice skin. J Dermatol Sci 86:238–248. https://doi.org/10.1016/j.jdermsci.2017.03.004
Sun WX, Liu Y, Zhou W et al (2017) Shikonin inhibits TNF-α production through suppressing PKC-NF-κB-dependent decrease of IL-10 in rheumatoid arthritis-like cell model. J Nat Med 71:349–356. https://doi.org/10.1007/s11418-016-1064-3
Kim H, Koh G (2000) Lipopolysaccharide activates matrix metalloproteinase-2 in endothelial cells through an NF-kappaB-dependent pathway. Biochem Biophys Res Commun 269:401–405. https://doi.org/10.1006/bbrc.2000.2308
Kowluru RA, Santos JM, Zhong Q (2014) Sirt1, a negative regulator of matrix metalloproteinase-9 in diabetic retinopathy. Invest Ophthalmol Vis Sci 55:5653–5660. https://doi.org/10.1167/iovs.14-14874
Sapieha P, Hamel D, Shao Z et al (2010) Proliferative retinopathies: angiogenesis that blinds. Int J Biochem Cell Biol 42:5–12. https://doi.org/10.1016/j.biocel.2009.10.006
Goukassian DA, Qin G, Dolan C et al (2007) Tumor necrosis factor-alpha receptor p75 is required in ischemia-induced neovascularization. Circulation 115:752–762. https://doi.org/10.1161/CIRCULATIONAHA.106.647255
Parra JL, Buxade M, Proud CG (2005) Features of the catalytic domains and C termini of the MAPK signal-integrating kinases Mnk1 and Mnk2 determine their differing activities and regulatory properties. J Biol Chem 280:37623–37633. https://doi.org/10.1074/jbc.M508356200
Horiuchi T, Mitoma H, Harashima S et al (2010) Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford) 49:1215–1228. https://doi.org/10.1093/rheumatology/keq031
Eissner G, Kolch W, Scheurich P (2004) Ligands working as receptors: reverse signaling by members of the TNF superfamily enhance the plasticity of the immune system. Cytokine Growth Factor Rev 15:353–366. https://doi.org/10.1016/j.cytogfr.2004.03.011
Su T, Zhong Y, Demetriades AM et al (2018) Endocan blockade suppresses experimental ocular neovascularization in mice. Invest Ophthalmol Vis Sci 59:930–939. https://doi.org/10.1167/iovs.17-22945
Coffelt SB, Hughes R, Lewis CE (2009) Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta 1796(1):11–18. https://doi.org/10.1016/j.bbcan.2009.02.004
Madan A, Penn JS (2003) Animal models of oxygen-induced retinopathy. Front Biosci 8:d1030–d1043. https://doi.org/10.2741/1056
Acknowledgments
The authors appreciate Shanghai Burn Research Institute for providing us with experimental facilities and the site.
Funding
Source of support: supported by the National Natural Science Foundation of China (No. 81570853).
Author information
Authors and Affiliations
Contributions
Conceptualization: Bing Xie and Xi Shen; methodology: Yixuan Yao, Cai Yujuan, Ailing Sui; formal analysis and investigation: Yixuan Yao; writing - original draft preparation: Yixuan Yao; writing - review and editing: Yixuan Yao, Yiyun Yao, Ting Su and Yanji Zhu; funding acquisition: Bing Xie.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Animal models and ethical approval
All animal-related procedures described here were in accordance with the National Institutes of Health Guide for the Use and Care of Laboratory Animals. The experiment protocol was approved by the Animal Care and Use Committee of Shanghai Jiao Tong University School of Medicine. Animals used in this study were specific pathogen-free C57BL/6 mice. We ensured to minimize animal suffering and the number of animals used.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yao, Y., Cai, Y., Sui, A. et al. Etanercept as a TNF-alpha inhibitor depresses experimental retinal neovascularization. Graefes Arch Clin Exp Ophthalmol 259, 661–671 (2021). https://doi.org/10.1007/s00417-020-04956-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-04956-6