Abstract
Purpose
To determine the vitreous levels of vascular endothelial growth factor (VEGF), stromal cell–derived factor-1α (SDF-1α) and angiopoietin-like protein 2 (ANGPTL2) in patients with active proliferative diabetic retinopathy (PDR), and to ascertain their contribution on different clinical presentation of active PDR.
Methods
This case–control study included 31 eyes with active PDR and 10 eyes with idiopathic macular hole (MH) (control group). Eyes with active PDR were divided into three subgroups: vitreous hemorrhage (VH), tractional retinal detachment (TRD) caused by active fibrovascular membrane (FVM), and coexistence of VH and TRD with FVM. Vitreous samples obtained during vitrectomy were analyzed for concentrations of VEGF, SDF-1α, and ANGPTL2.
Results
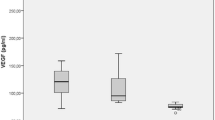
Vitreous level of VEGF (2021 (168–6550) pg/ml vs 110.1 (74.5–236) pg/ml), SDF-1α (517 (194–1044) pg/ml vs 388 (320–535) pg/ml), and ANGPTL2 (725 (131–1590) ng/ml vs 196 (75.9–437) ng/ml) were significantly higher in eyes with active PDR than in control group (p < 0.001, p = 0.002, and p < 0.001, respectively). The concentrations of these meaditors in each active PDR subgroups were also significantly higher than control group (p < 0.05). The vitreous level of ANGPTL2 was significantly higher in eyes with TRD caused by FVM (1033 ± 401 ng/ml) than in eyes with VH (561 ± 237 ng/ml; p = 0.008).
Conclusion
High levels of SDF-1α, ANGPTL2 and particularly VEGF seem to be associated with PDR. Since the vitreous levels of ANGPTL2 tend to be higher in eyes with active fibrovascular tractional detachment, vitreous levels of this chemokine seem to be affected by the clinical presentation of vascularly active PDR eyes.


Similar content being viewed by others
References
Prokofyeva E, Zrenner E (2012) Epidemiology of major eye diseases leading to blindness in Europe: a literature review. Ophthalmic Res 47:171–188. https://doi.org/10.1159/000329603
Nentwich MM, Ulbig MW (2015) Diabetic retinopathy—ocular complications of diabetes mellitus. World J Diabetes 6:489–499. https://doi.org/10.4239/wjd.v6.i3.489
Stitt AW (2010) AGEs and diabetic retinopathy. Investig Ophthalmol Vis Sci 51:4867–4874. https://doi.org/10.1167/iovs.10-5881
Durham JT, Herman IM (2011) Microvascular modifications in diabetic retinopathy. Curr Diab Rep 11:253–264. https://doi.org/10.1007/s11892-011-0204-0
Pepper MS (1996) Positive and negative regulation of angiogenesis: from cell biology to the clinic. Vasc Med 1:259–266. https://doi.org/10.1177/1358863X9600100404
Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO (2003) Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 22:1–29. https://doi.org/10.1016/s1350-9462(02)00043-5
Holmes DI, Zachary I (2005) The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol 6:209. https://doi.org/10.1186/gb-2005-6-2-209
Byrne AM, Bouchier-Hayes DJ, Harmey JH (2005) Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med 9:777–794. https://doi.org/10.1111/j.1582-4934.2005.tb00379.x
Dreyfuss JL, Giordano RJ, Regatieri CV (2015) Ocular angiogenesis. J Ophthalmol 2015:892043. https://doi.org/10.1155/2015/892043
Jones CD, Greenwood RH, Misra A, Bachmann MO (2012) Incidence and progression of diabetic retinopathy during 17 years of a population-based screening program in England. Diabetes Care 35:592–596. https://doi.org/10.2337/dc11-0943
Dutra Medeiros M, Mesquita E, Gardete-Correia L, Moita J, Genro V, Papoila AL, Amaral-Turkman A, Raposo JF (2015) First incidence and progression study for diabetic retinopathy in Portugal, the RETINODIAB study: evaluation of the screening program for Lisbon region. Ophthalmology 122:2473–2481. https://doi.org/10.1016/j.ophtha.2015.08.004
Klein R, Klein BE, Moss SE, Davis MD, DeMets DL (1989) The Wisconsin epidemiologic study of diabetic retinopathy. X. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Arch Ophthalmol 107:244–249. https://doi.org/10.1001/archopht.1989.01070010250031
Mahdy RA, Nada WM, Hadhoud KM, El-Tarhony SA (2010) The role of vascular endothelial growth factor in the progression of diabetic vascular complications. Eye (Lond) 24:1576–1584. https://doi.org/10.1038/eye.2010.86
Al Kahtani E, Xu Z, Al Rashaed S et al (2017) Vitreous levels of placental growth factor correlate with activity of proliferative diabetic retinopathy and are not influenced by bevacizumab treatment. Eye (Lond) 31:529–536. https://doi.org/10.1038/eye.2016.246
Wu F, Phone A, Lamy R et al (2020) Correlation of aqueous, vitreous, and plasma cytokine levels in patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 61:26. https://doi.org/10.1167/iovs.61.2.26
Mesquita J, Castro-de-Sousa JP, Vaz-Pereira S, Neves A, Passarinha LA, Tomaz CT (2018) Evaluation of the growth factors VEGF-a and VEGF-B in the vitreous and serum of patients with macular and retinal vascular diseases. Growth Factors 36:48–57. https://doi.org/10.1080/08977194.2018.1477140
Chernykh VV, Varvarinsky EV, Smirnov EV, Chernykh DV, Trunov AN (2015) Proliferative and inflammatory factors in the vitreous of patients with proliferative diabetic retinopathy. Indian J Ophthalmol 63:33–36. https://doi.org/10.4103/0301-4738.151464
Korobelnik JF, Do DV, Schmidt-Erfurth U et al (2014) Intravitreal aflibercept for diabetic macular edema. Ophthalmology 121:2247–2254. https://doi.org/10.1016/j.ophtha.2014.05.006
Massin P, Bandello F, Garweg JG et al (2010) Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care 33:2399–2405. https://doi.org/10.2337/dc10-0493
Ip MS, Domalpally A, Sun JK, Ehrlich JS (2015) Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology 122:367–374. https://doi.org/10.1001/archophthalmol.2012.1043
You JJ, Yang CH, Huang JS, Chen MS, Yang CM (2007) Fractalkine, a CX3C chemokine, as a mediator of ocular angiogenesis. Invest Ophthalmol Vis Sci 48:5290–5298. https://doi.org/10.1167/iovs.07-0187
Mitamura Y, Tashimo A, Nakamura Y, Tagawa H, Ohtsuka K, Mizue Y, Nishihira J (2002) Vitreous levels of placenta growth factor and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Diabetes Care 25:2352. https://doi.org/10.2337/diacare.25.12.2352
Aiello LP, Avery RL, Arrigg PG et al (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331:1480–1487. https://doi.org/10.1056/NEJM199412013312203
Song Z, Sun M, Zhou F, Qu J, Chen D (2014) Increased intravitreous interleukin-18 correlated to vascular endothelial growth factor in patients with active proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 252:1229–1234. https://doi.org/10.1007/s00417-014-2586-6
Salcedo R, Oppenheim JJ (2003) Role of chemokines in angiogenesis: CXCL12/SDF-1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation 10:359–370. https://doi.org/10.1038/sj.mn.7800200
Sonmez K, Drenser KA, Capone A Jr, Trese MT (2008) Vitreous levels of stromal cell-derived factor 1 and vascular endothelial growth factor in patients with retinopathy of prematurity. Ophthalmology 115:1065–1070. https://doi.org/10.1016/j.ophtha.2007.08.050
Chen LY, Zhuo YH, Li YH, Huang XH, Zhang JL, Li SY, Wang XG, Lü L (2010) Expression of stromal cell-derived factor-1 in diabetic retinopathy. Chin Med J 123:984–988
Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME (2008) Vascular endothelial growth factor in eye disease. Prog Retin Eye Res 27:331–371. https://doi.org/10.1016/j.preteyeres.2008.05.001
Ito Y, Oike Y, Tanihara H (2013) Angiopoietin-like protein 2 contributes to pathogenesis of diabetic retinopathy. Acta Opthalmologica 91:s252. https://doi.org/10.1111/j.1755-3768.2013.T082.x
Sasaki Y, Ohta M, Desai D et al (2015) Angiopoietin like protein 2 (ANGPTL2) promotes adipose tissue macrophage and T lymphocyte accumulation and leads to insulin resistance. PLoS One 10:e0131176. https://doi.org/10.1371/journal.pone.0131176
Horio E, Kadomatsu T, Miyata K et al (2014) Role of endothelial cell-derived ANGPTL2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arterioscler Thromb Vasc Biol 34:790–800. https://doi.org/10.1161/ATVBAHA.113.303116
Farhat N, Thorin-Trescases N, Mamarbachi M et al (2013) Angiopoietin-like 2 promotes atherogenesis in mice. J Am Heart Assoc 2:e000201. https://doi.org/10.1161/JAHA.113.000201
Tabata M, Kadomatsu T, Fukuhara S et al (2009) Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab 10:178–188. https://doi.org/10.1016/j.cmet.2009.08.003
Okada T, Tsukano H, Endo M et al (2010) Synoviocyte-derived angiopoietin-like protein 2 contributes to synovial chronic inflammation in rheumatoid arthritis. Am J Pathol 176:2309–2319. https://doi.org/10.2353/ajpath.2010.090865
Aoi J, Endo M, Kadomatsu T et al (2011) Angiopoietin-like protein 2 is an important facilitator of inflammatory carcinogenesis and metastasis. Cancer Res 71:7502–7512. https://doi.org/10.1158/0008-5472.CAN-11-1758
Endo M, Nakano M, Kadomatsu T et al (2012) Tumor cell-derived angiopoietin-like protein ANGPTL2 is a critical driver of metastasis. Cancer Res 72:1784–1794. https://doi.org/10.1158/0008-5472.CAN-11-3878
Doi Y, Ninomiya T, Hirakawa Y et al (2013) Angiopoietin-like protein 2 and risk of type 2 diabetes in a general Japanese population: the Hisayama study. Diabetes Care 36:98–100. https://doi.org/10.2337/dc12-0166
Gellen B, Thorin-Trescases N, Sosner P et al (2016) ANGPTL2 is associated with an increased risk of cardiovascular events and death in diabetic patients. Diabetologia 59:2321–2330. https://doi.org/10.1007/s00125-016-4066-5
Hato T, Tabata M, Oike Y (2008) The role of angiopoietin-like proteins in angiogenesis and metabolism. Trends Cardiovasc Med 18:6–14. https://doi.org/10.1016/j.tcm.2007.10.003
Kadomatsu T, Endo M, Miyata K, Oike Y (2014) Diverse roles of ANGPTL2 in physiology and pathophysiology. Trends Endocrinol Metab 25:245–254. https://doi.org/10.1016/j.tem.2014.03.012
Van Geest RJ, Lesnik-Oberstein SY, Tan HS, Mura M, Goldschmeding R, VanNoorden CJ, Klaassen I, Schlingemann RO (2012) A shift in the balance of vascular endothelial growth factor and connective tissue growth factor by bevacizumab causes the angiofibrotic switch in proliferative diabetic retinopathy. Br J Ophthalmol 96:587–590. https://doi.org/10.1136/bjophthalmol-2011-301005
Ogata N, Nishikawa M, Nishimura T, Mitsuma Y, Matsumura M (2002) Unbalanced vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor in diabetic retinopathy. Am J Ophthalmol 134:348–353. https://doi.org/10.1016/s0002-9394(02)01568-4
Author information
Authors and Affiliations
Contributions
Design of the study (KS, AK), conduct of the study (AK, KS, and YOE), analysis and interpretation (AK, KS, YOE, SNA, and EO), and literature search (AK, KS, and YOE).
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Ethics approval
The study adhered to the tenets of Declaration of Helsinki and was approved by the Institutional Review Board/Ethics Committee of Numune Training and Research Hospital, Ankara, Turkey (protocol no: E-17-1405).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Keles, A., Sonmez, K., Erol, Y.O. et al. Vitreous levels of vascular endothelial growth factor, stromal cell–derived factor-1α, and angiopoietin-like protein 2 in patients with active proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 259, 53–60 (2021). https://doi.org/10.1007/s00417-020-04889-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-04889-0