Abstract
Purpose
Human corneal epithelial cell-transformed (HCE-T) cell line is used as a widely accepted barrier model for pharmacological investigations in the context of eye application. The differentiation of (limbal) corneal epithelial into mature corneal epithelium coincides with the expression of established differentiation markers. If these differentiation mechanisms are disturbed, it will lead to ocular surface disease. In this study, we want to compare the expression of differentiation markers in the HCE-T cell line to differentiated primary epithelial cells (pCECs) and primary limbal epithelial cell (LEC) culture. This is necessary in order to decide whether HCE-T cells could be a tool to study the differentiation process and its regulatory networks in corneal epithelium.
Methods
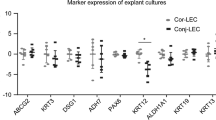
Primary limbal epithelial cells (LECs) for cell culture and primary corneal epithelial cells (pCECs) as differentiated tissue samples were obtained from the limbus or central cornea region of corneal donors. HCE-T cell line was purchased from RIKEN Institute RCB-2280.Expression levels of conjunctival- and corneal-specific keratin and adhesion markers (KRT3, KRT12, KRT13, KRT19, DSG1), stem cell and differentiation markers (PAX6, ABCG2, ADH7, TP63, ALDH1A1), and additional (unvalidated) putative differentiation and stem cell markers (CTSV, SPINK7, DKK1) were analyzed with qPCR. Additionally, KRT3, KRT12, DSG1, and PAX6 protein levels were analyzed with Western blot.
Results
KRT3, KRT12, DSG1, PAX6, ADH7, and ALDH1A1 mRNA expressions were higher in LECs and magnitudes higher in pCECs compared to HCE-T cells. KRT3, KRT12, PAX6, ALDH1A1, ADH7, TP63, and CTSV mRNAs have shown increasing mRNA expression from HCE-T < HCE-T cultured in keratinocyte serum-free medium (KSFM) < LEC < to pCEC.KRT3 and KRT12 protein expressions were only slightly increased in LEC compared to HCE-T samples, and the strongest signals were seen in pCEC samples. DSG1 protein expression was only detected in pCECs. PAX6 protein expression was hardly detected in HCE-T cells, and no difference could be seen between LECs and pCECs.
Conclusions
The HCE-T cell line is even less differentiated than LECs regarding the investigated markers and therefore might also lack the ability to express differentiation markers at protein level. Hence, this cell line is not suitable to study corneal differentiation processes. Primary LECs in the way cultured here are not an ideal system compared to differentiated epithelium in organ culture but should be preferred to HCE-T cells if corneal differentiation markers are investigated. Other cell models or differentiation protocols should be developed in the future to gain new tools for research on ocular surface diseases.




Similar content being viewed by others
References
Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H (1995) An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci 36(3):614–621
Reichl S (2008) Cell culture models of the human cornea - a comparative evaluation of their usefulness to determine ocular drug absorption in-vitro. J Pharm Pharmacol 60(3):299–307. https://doi.org/10.1211/jpp.60.3.0004
Becker U, Ehrhardt C, Schneider M, Muys L, Gross D, Eschmann K, Schaefer UF, Lehr CM (2008) A comparative evaluation of corneal epithelial cell cultures for assessing ocular permeability. Altern Lab Anim 36(1):33–44
Toropainen E, Ranta V-P, Talvitie A, Suhonen P, Urtti A (2001) Culture model of human corneal epithelium for prediction of ocular drug absorption. Invest Ophthalmol Vis Sci 42(12):2942–2948
Juretic M, Jurisic Dukovski B, Krtalic I, Reichl S, Cetina-Cizmek B, Filipovic-Grcic J, Lovric J, Pepic I (2017) HCE-T cell-based permeability model: a well-maintained or a highly variable barrier phenotype? Eur J Pharm Sci 104:23–30. https://doi.org/10.1016/j.ejps.2017.03.018
Ronkko S, Vellonen KS, Jarvinen K, Toropainen E, Urtti A (2016) Human corneal cell culture models for drug toxicity studies. Drug Deliv Transl Res 6(6):660–675. https://doi.org/10.1007/s13346-016-0330-y
Yamasaki K, Kawasaki S, Young RD, Fukuoka H, Tanioka H, Nakatsukasa M, Quantock AJ, Kinoshita S (2009) Genomic aberrations and cellular heterogeneity in SV40-immortalized human corneal epithelial cells. Invest Ophthalmol Vis Sci 50(2):604–613. https://doi.org/10.1167/iovs.08-2239
Kolln C, Reichl S (2012) mRNA expression of metabolic enzymes in human cornea, corneal cell lines, and hemicornea constructs. J Ocul Pharmacol Ther 28(3):271–277. https://doi.org/10.1089/jop.2011.0124
Becker U, Ehrhardt C, Daum N, Baldes C, Schaefer UF, Ruprecht KW, Kim KJ, Lehr CM (2007) Expression of ABC-transporters in human corneal tissue and the transformed cell line, HCE-T. J Ocul Pharmacol Ther 23(2):172–181. https://doi.org/10.1089/jop.2006.0095
Verstraelen J, Reichl S (2014) Multidrug resistance-associated protein (MRP1, 2, 4 and 5) expression in human corneal cell culture models and animal corneal tissue. Mol Pharm 11(7):2160–2171. https://doi.org/10.1021/mp400625z
Ho JH, Chuang CH, Ho CY, Shih YR, Lee OK, Su Y (2007) Internalization is essential for the antiapoptotic effects of exogenous thymosin beta-4 on human corneal epithelial cells. Invest Ophthalmol Vis Sci 48(1):27–33. https://doi.org/10.1167/iovs.06-0826
Ho JH, Tseng KC, Ma WH, Chen KH, Lee OK, Su Y (2008) Thymosin beta-4 upregulates anti-oxidative enzymes and protects human cornea epithelial cells against oxidative damage. Br J Ophthalmol 92(7):992–997. https://doi.org/10.1136/bjo.2007.136747
Hogerheyde TA, Stephenson SA, Harkin DG, Bray LJ, Madden PW, Woolf MI, Richardson NA (2013) Evaluation of Eph receptor and ephrin expression within the human cornea and limbus. Exp Eye Res 107:110–120. https://doi.org/10.1016/j.exer.2012.11.016
Klinngam W, Fu R, Janga SR, Edman MC, Hamm-Alvarez SF (2018) Cathepsin S alters the expression of pro-inflammatory cytokines and MMP-9, partially through protease-activated receptor-2, in human corneal epithelial cells. Int J Mol Sci 19(11). https://doi.org/10.3390/ijms19113530
Kurpakus MA, Daneshvar C, Davenport J, Kim A (1999) Human corneal epithelial cell adhesion to laminins. Curr Eye Res 19(2):106–114
Lang R, Song PI, Legat FJ, Lavker RM, Harten B, Kalden H, Grady EF, Bunnett NW, Armstrong CA, Ansel JC (2003) Human corneal epithelial cells express functional PAR-1 and PAR-2. Invest Ophthalmol Vis Sci 44(1):99–105
Nagai N, Fukuoka Y, Ishii M, Otake H, Yamamoto T, Taga A, Okamoto N, Shimomura Y (2018) Instillation of sericin enhances corneal wound healing through the ERK pathway in rat debrided corneal epithelium. Int J Mol Sci 19(4). https://doi.org/10.3390/ijms19041123
Nagai N, Inomata M, Ito Y (2008) Contribution of aldehyde dehydrogenase 3A1 to disulfiram penetration through monolayers consisting of cultured human corneal epithelial cells. Biol Pharm Bull 31(7):1444–1448
Seomun Y, Joo CK (2008) Lumican induces human corneal epithelial cell migration and integrin expression via ERK 1/2 signaling. Biochem Biophys Res Commun 372(1):221–225. https://doi.org/10.1016/j.bbrc.2008.05.014
Tong L, Png E, Aihua H, Yong SS, Yeo HL, Riau A, Mendoz E, Chaurasia SS, Lim CT, Yiu TW, Iismaa SE (2013) Molecular mechanism of transglutaminase-2 in corneal epithelial migration and adhesion. Biochim Biophys Acta 1833(6):1304–1315. https://doi.org/10.1016/j.bbamcr.2013.02.030
Verstraelen J, Reichl S (2015) Upregulation of P-glycoprotein expression by ophthalmic drugs in different corneal in-vitro models. J Pharm Pharmacol 67(5):605–615. https://doi.org/10.1111/jphp.12357
Wang M, Munier F, Araki-Saski K, Schorderet D (2002) TGFBI gene transcript is transforming growth factor-beta1-responsive and cell density-dependent in a human corneal epithelial cell line. Ophthalmic Genet 23(4):237–245
Yamada T, Ueda T, Ugawa S, Ishida Y, Imayasu M, Koyama S, Shimada S (2010) Functional expression of transient receptor potential vanilloid 3 (TRPV3) in corneal epithelial cells: involvement in thermosensation and wound healing. Exp Eye Res 90(1):121–129. https://doi.org/10.1016/j.exer.2009.09.020
Zimowska G, Shi J, Munguba G, Jackson MR, Alpatov R, Simmons MN, Shi Y, Sugrue SP (2003) Pinin/DRS/memA interacts with SRp75, SRm300 and SRrp130 in corneal epithelial cells. Invest Ophthalmol Vis Sci 44(11):4715–4723
Greco D, Vellonen KS, Turner HC, Hakli M, Tervo T, Auvinen P, Wolosin JM, Urtti A (2010) Gene expression analysis in SV-40 immortalized human corneal epithelial cells cultured with an air-liquid interface. Mol Vis 16:2109–2120
Schermer A, Galvin S, Sun TT (1986) Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol 103(1):49–62
Ramirez-Miranda A, Nakatsu MN, Zarei-Ghanavati S, Nguyen CV, Deng SX (2011) Keratin 13 is a more specific marker of conjunctival epithelium than keratin 19. Mol Vis 17:1652–1661
Ramos T, Scott D, Ahmad S (2015) An update on ocular surface epithelial stem cells: cornea and conjunctiva. Stem Cells Int 2015:601731. https://doi.org/10.1155/2015/601731
Davis J, Duncan MK, Robison WG Jr, Piatigorsky J (2003) Requirement for Pax6 in corneal morphogenesis: a role in adhesion. J Cell Sci 116(Pt 11):2157–2167. https://doi.org/10.1242/jcs.00441
Turner HC, Budak MT, Akinci MA, Wolosin JM (2007) Comparative analysis of human conjunctival and corneal epithelial gene expression with oligonucleotide microarrays. Invest Ophthalmol Vis Sci 48(5):2050–2061. https://doi.org/10.1167/iovs.06-0998
Liu JJ, Kao WW, Wilson SE (1999) Corneal epithelium-specific mouse keratin K12 promoter. Exp Eye Res 68(3):295–301. https://doi.org/10.1006/exer.1998.0593
Ramaesh K, Ramaesh T, Dutton GN, Dhillon B (2005) Evolving concepts on the pathogenic mechanisms of aniridia related keratopathy. Int J Biochem Cell Biol 37(3):547–557. https://doi.org/10.1016/j.biocel.2004.09.002
Li G, Xu F, Zhu J, Krawczyk M, Zhang Y, Yuan J, Patel S, Wang Y, Lin Y, Zhang M, Cai H, Chen D, Zhang M, Cao G, Yeh E, Lin D, Su Q, W-w L, Sen GL, Afshari N, Chen S, Maas RL, Fu X-D, Zhang K, Liu Y, Ouyang H (2015) Transcription factor PAX6 (paired box 6) controls limbal stem cell lineage in development and disease. J Biol Chem 290(33):20448–20454. https://doi.org/10.1074/jbc.M115.662940
Budak MT, Alpdogan OS, Zhou M, Lavker RM, Akinci MA, Wolosin JM (2005) Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J Cell Sci 118(Pt 8):1715–1724. https://doi.org/10.1242/jcs.02279
Satre MA, Zgombic-Knight M, Duester G (1994) The complete structure of human class IV alcohol dehydrogenase (retinol dehydrogenase) determined from the ADH7 gene. J Biol Chem 269(22):15606–15612
Ebrahimi M, Taghi-Abadi E, Baharvand H (2009) Limbal stem cells in review. J Ophthalmic Vis Res 4(1):40–58
Forsdahl S, Kiselev Y, Hogseth R, Mjelle JE, Mikkola I (2014) Pax6 regulates the expression of Dkk3 in murine and human cell lines, and altered responses to Wnt signaling are shown in FlpIn-3T3 cells stably expressing either the Pax6 or the Pax6(5a) isoform. PLoS One 9(7):e102559. https://doi.org/10.1371/journal.pone.0102559
Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M (2001) p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A 98(6):3156–3161. https://doi.org/10.1073/pnas.061032098
Labrecque J, Dumas F, Lacroix A, Bhat PV (1995) A novel isoenzyme of aldehyde dehydrogenase specifically involved in the biosynthesis of 9-cis and all-trans retinoic acid. Biochem J 305(Pt 2):681–684
Kumar S, Dollé P, Ghyselinck NB, Duester G (2017) Endogenous retinoic acid signaling is required for maintenance and regeneration of cornea. Exp Eye Res 154:190–195. https://doi.org/10.1016/j.exer.2016.11.009
Adachi W, Kawamoto S, Ohno I, Nishida K, Kinoshita S, Matsubara K, Okubo K (1998) Isolation and characterization of human cathepsin V: a major proteinase in corneal epithelium. Invest Ophthalmol Vis Sci 39(10):1789–1796
Furio L, Hovnanian A (2011) When activity requires breaking up: LEKTI proteolytic activation cascade for specific proteinase inhibition. J Invest Dermatol 131(11):2169–2173. https://doi.org/10.1038/jid.2011.295
Meyer-Hoffert U, Wu Z, Kantyka T, Fischer J, Latendorf T, Hansmann B, Bartels J, He Y, Glaser R, Schroder JM (2010) Isolation of SPINK6 in human skin: selective inhibitor of kallikrein-related peptidases. J Biol Chem 285(42):32174–32181. https://doi.org/10.1074/jbc.M109.091850
Nakatsu MN, Ding Z, Ng MY, Truong TT, Yu F, Deng SX (2011) Wnt/β-catenin signaling regulates proliferation of human cornea epithelial stem/progenitor cells. Invest Ophthalmol Vis Sci 52(7):4734–4741. https://doi.org/10.1167/iovs.10-6486
Schlotzer-Schrehardt U, Kruse FE (2005) Identification and characterization of limbal stem cells. Exp Eye Res 81(3):247–264. https://doi.org/10.1016/j.exer.2005.02.016
Secker GA, Daniels JT (2008) Corneal epithelial stem cells: deficiency and regulation. Stem Cell Rev 4(3):159–168. https://doi.org/10.1007/s12015-008-9029-x
Kurpakus MA, Stock EL, Jones JC (1990) Expression of the 55-kD/64-kD corneal keratins in ocular surface epithelium. Invest Ophthalmol Vis Sci 31(3):448–456
Bath C, Muttuvelu D, Emmersen J, Vorum H, Hjortdal J, Zachar V (2013) Transcriptional dissection of human limbal niche compartments by massive parallel sequencing. PLoS One 8(5):e64244–e64244. https://doi.org/10.1371/journal.pone.0064244
Blumenberg M (2006) Transcriptional regulation of keratin gene expression. In: Intermediate Filaments. Springer US, Boston, pp 93–109. https://doi.org/10.1007/0-387-33781-4_7
Di Iorio E, Barbaro V, Ruzza A, Ponzin D, Pellegrini G, De Luca M (2005) Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci U S A 102(27):9523–9528. https://doi.org/10.1073/pnas.0503437102
Sasamoto Y, Hayashi R, Park S-J, Saito-Adachi M, Suzuki Y, Kawasaki S, Quantock AJ, Nakai K, Tsujikawa M, Nishida K (2016) PAX6 isoforms, along with reprogramming factors, differentially regulate the induction of cornea-specific genes. Sci Rep 6:20807. https://doi.org/10.1038/srep20807 http://www.nature.com/articles/srep20807#supplementary-information
Kiselev Y, Eriksen TE, Forsdahl S, Nguyen LH, Mikkola I (2012) 3T3 cell lines stably expressing Pax6 or Pax6(5a)—a new tool used for identification of common and isoform specific target genes. PLoS One 7(2):e31915. https://doi.org/10.1371/journal.pone.0031915
Latta L, Viestenz A, Stachon T, Colanesi S, Szentmáry N, Seitz B, Käsmann-Kellner B (2018) Human aniridia limbal epithelial cells lack expression of keratins K3 and K12. Exp Eye Res 167 (Supplement C):100–109 doi:https://doi.org/10.1016/j.exer.2017.11.005
Li W, Chen YT, Hayashida Y, Blanco G, Kheirkah A, He H, Chen SY, Liu CY, Tseng SC (2008) Down-regulation of Pax6 is associated with abnormal differentiation of corneal epithelial cells in severe ocular surface diseases. J Pathol 214(1):114–122. https://doi.org/10.1002/path.2256
Kitazawa K, Hikichi T, Nakamura T, Sotozono C, Kinoshita S, Masui S (2017) PAX6 regulates human corneal epithelium cell identity. Exp Eye Res 154:30–38. https://doi.org/10.1016/j.exer.2016.11.005
Roux IP, Romain D, Jean-Paul C, Jieqiong Q, Huiqing Z, Alain J, Olivier F, Daniel A (2018) Modeling of aniridia-related keratopathy by CRISPR/Cas9 genome editing of human limbal epithelial cells and rescue by recombinant PAX6 protein. Stem Cells 36(9). https://doi.org/10.1002/stem.2858
Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE (2008) Structure and function of claudins. Biochim Biophys Acta 1778(3):631–645. https://doi.org/10.1016/j.bbamem.2007.10.018
Acknowledgments
This work was supported by The Dr. Rolf M. Schwiete Foundation and HOMFOR.
The authors thank Prof. Flockerzi, Department of Experimental and Clinical Pharmacology and Toxicology, for providing the Western blot imaging system and Nanodrop. They would also like to thank Prof. Hoth, Department of Biophysics, Saarland University, for providing the qPCR system.
Funding
This study was funded by The Rolf M. Schwiete Stiftung and HOMFOR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM1
(PDF 1.37 mb)
Rights and permissions
About this article
Cite this article
Rubelowski, AK., Latta, L., Katiyar, P. et al. HCE-T cell line lacks cornea-specific differentiation markers compared to primary limbal epithelial cells and differentiated corneal epithelium. Graefes Arch Clin Exp Ophthalmol 258, 565–575 (2020). https://doi.org/10.1007/s00417-019-04563-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04563-0