Abstract
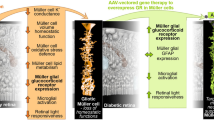
Diabetic retinopathy (DR) is a sight-threatening complication associated with the highly prevalent diabetes disorder. Both the microvascular damage and neurodegeneration detected in the retina caused by chronic hyperglycemia have brought special attention to Müller cells, the major macroglia of the retina that are responsible for retinal homeostasis. Given the role of glucocorticoid signaling in anti-inflammatory responses and the almost exclusive expression of glucocorticoid receptors (GRs) in retinal Müller cells, administration of corticosteroid agonists as a potential treatment option has been widely studied. Although these approaches have been moderately efficacious in treating or de-escalating DR pathomechanisms, there are various side effects and gaps of knowledge with regard to introducing exogenous glucocorticoids to the diseased retina. In this paper, we provide a review of the literature concerning the available evidence for the role of Müller cell glucocorticoid signaling in DR and we discuss previously investigated approaches in modulating this system as possible treatment options. Furthermore, we propose a novel alternative to the available choices of treatment by using gene therapy as a tool to regulate the expression of GR in retinal Müller cells. Upregulating GR expression allows for induced glucocorticoid signaling with more enduring effects compared to injection of agonists. Hence, repetitive injections would no longer be required. Lastly, side effects of glucocorticoid therapy such as glucocorticoid resistance of GR following chronic exposure to excess ligands or agonists can be avoided.


Similar content being viewed by others
References
Emerging Risk Factors Collaboration (2010) Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375(9733):2215–2222
Yau JW, Rogers SL, Kawasaki R et al (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35(3):556–564
Lee R, Wong TY, Sabanayagam C (2015) Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vision 2(1):17
Olivares AM, Althoff K, Chen GF et al (2017) Animal models of diabetic retinopathy. Curr Diabetes Rep 17(10):93
Hammes HP (2018) Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia 61(1):29–38
Tuomi T (2005) Type 1 and type 2 diabetes: what do they have in common? Diabetes. 54(suppl 2):S40–S45
Sayin N, Kara N, Pekel G (2015) Ocular complications of diabetes mellitus. World J Diabetes 6(1):92
Lachin JM, Genuth S, Nathan DM (2008) Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial—revisited. Diabetes 57(4):995–1001
Zong H, Ward M, Stitt AW (2011) AGEs, RAGE, and diabetic retinopathy. Curr Diabetes Rep 11(4):244–252
Forbes JM, Cooper ME (2013) Mechanisms of diabetic complications. Physiol Rev 93(1):137–188
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107(9):1058–1070
Madsen-Bouterse SA, Kowluru RA (2008) Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord 9(4):315–327
Thornalley PJ (2003) Glyoxalase I—structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans 31:1343–1348
Rabbani N, Xue M, Thornalley PJ (2016) Dicarbonyls and glyoxalase in disease mechanisms and clinical therapeutics. Glycoconj J 33(4):513–525
Hidmark A, Fleming T, Vittas S (2014) A new paradigm to understand and treat diabetic neuropathy. Exp Clin Endocrinol Diabetes 226(04):201–207
Sachdeva R, Schlotterer A, Schumacher D (2018) TRPC proteins contribute to development of diabetic retinopathy and regulate glyoxalase 1 activity and methylglyoxal accumulation. Mol Metab 9:156–167
Malaguarnera L, Zorena K (2016) Neurodegeneration and neuroinflammation in diabetic retinopathy: potential approaches to delay neuronal loss. Curr Neuropharmacol 14(8):831–839
Reiter CE, Gardner TW (2003) Functions of insulin and insulin receptor signaling in retina: possible implications for diabetic retinopathy. Prog Retin Eye Res 22(4):545–562
Barber AJ, Lieth E, Khin SA et al (1998) Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest 102(4):783–791
Fort PE, Losiewicz MK, Reiter CE et al (2011) Differential roles of hyperglycemia and hypoinsulinemia in diabetes induced retinal cell death: evidence for retinal insulin resistance. PLoS One 6(10):e26498
Duh EJ, Sun JK, Stitt AW (2017) Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI insight 2(14)
Zhang X, Zeng H, Bao S et al (2014) Diabetic macular edema: new concepts in patho-physiology and treatment. Cell Biosci 4(1):27
Kim YW, Byzova TV (2014) Oxidative stress in angiogenesis and vascular disease. Blood 123(5):625–631
Arjamaa O, Nikinmaa M (2006) Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp Eye Res 83(3):473–483
Gupta N, Mansoor S, Sharma A et al (2013) Diabetic retinopathy and VEGF. Open Ophthalmol J 7:4
Robinson R, Barathi VA, Chaurasia SS (2012) Update on animal models of diabetic retinopathy: from molecular approaches to mice and higher mammals. Dis Model Mech 5(4):444–456
Reichenbach A, Bringmann A (2013) New functions of Müller cells. Glia. 61(5):651–678
Coughlin BA, Feenstra DJ, Mohr S (2017) Müller cells and diabetic retinopathy. Vis Res 139:93–100
Reichenbach A, Wurm A, Pannicke T et al (2007) Müller cells as players in retinal degeneration and edema. Graefes Arch Clin Exp Ophthalmol 245(5):627–636
Lieth E, Barber AJ, Xu B et al (1998) Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes. 47(5):815–820
Kowluru RA, Engerman RL, Case GL et al (2001) Retinal glutamate in diabetes and effect of antioxidants. Neurochem Int 38(5):385–390
Li Q, Puro DG (2002) Diabetes-induced dysfunction of the glutamate transporter in retinal Muller cells. Invest Ophthalmol Vis Sci 43(9):3109–3116
Eichler W, Kuhrt H, Hoffmann S et al (2000) VEGF release by retinal glia depends on both oxygen and glucose supply. Neuroreport. 11(16):3533–3537
Pannicke T, Iandiev I, Wurm A et al (2006) Diabetes alters osmotic swelling characteristics and membrane conductance of glial cells in rat retina. Diabetes. 55(3):633–639
Newman E, Reichenbach A (1996) The Müller cell: a functional element of the retina. Trends Neurosci 19(8):307–312
Pannicke T, Iandiev I, Uckermann O et al (2004) A potassium channel-linked mechanism of glial cell swelling in the postischemic retina. Mol Cell Neurosci 26(4):493–502
Iandiev I, Tenckhoff S, Pannicke T et al (2006) Differential regulation of Kir4. 1 and Kir2. 1 expression in the ischemic rat retina. Neurosci Lett 396(2):97–101
Pannicke T, Uckermann O, Iandiev I et al (2005) Ocular inflammation alters swelling and membrane characteristics of rat Müller glial cells. J Neuroimmunol 161(1–2):145–154
Krügel K, Wurm A, Pannicke T et al (2011) Involvement of oxidative stress and mitochondrial dysfunction in the osmotic swelling of retinal glial cells from diabetic rats. Exp Eye Res 92(1):87–93
Wurm A, Iandiev I, Hollborn M et al (2008) Purinergic receptor activation inhibits osmotic glial cell swelling in the diabetic rat retina. Exp Eye Res 87(4):385–393
Pazdro R, Burgess JR (2010) The role of vitamin E and oxidative stress in diabetes complications. Mech Ageing Dev 131(4):276–286
Du Y, Sarthy VP, Kern TS (2004) Interaction between NO and COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. Am J Phys Regul Integr Comp Phys 287(4):R735–R741
Kino T (2017) Glucocorticoid receptor. https://www.ncbi.nlm.nih.gov/books/NBK279171/?report=reader. Accessed 14 Oct 2019.
Gallina D, Zelinka C, Fischer AJ (2014) Glucocorticoid receptors in the retina, Müller glia and the formation of Müller glia-derived progenitors. Development. 141(17):3340–3351
Schaaf MJ, Cidlowski JA (2002) Molecular mechanisms of glucocorticoid action and resistance. J Steroid Biochem Mol Biol 83(1–5):37–48
Zhang X, Wang N, Schachat AP et al (2014) Glucocorticoids: structure, signaling and molecular mechanisms in the treatment of diabetic retinopathy and diabetic macular edema. Curr Mol Med 14(3):376–384
Yeager MP, Pioli PA, Guyre PM (2011) Cortisol exerts bi-phasic regulation of inflammation in humans. Dose-Response. 9(3):332–347
Roy MS, Roy A, Brown S (1998) Increased urinary-free cortisol outputs in diabetic patients. J Diabetes Complicat 12(1):24–27
Chiodini I, Adda G, Scillitani A et al (2007) Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications. Diabetes Care 30(1):83–88
Erickson RL, Browne CA, Lucki I (2017) Hair corticosterone measurement in mouse models of type 1 and type 2 diabetes mellitus. Physiol Behav 178:166–171
Vandevyver S, Dejager L, Libert C (2014) Comprehensive overview of the structure and regulation of the glucocorticoid receptor. Endocr Rev 35(4):671–693
Gallina D, Zelinka CP, Cebulla CM et al (2015) Activation of glucocorticoid receptors in Müller glia is protective to retinal neurons and suppresses microglial reactivity. Exp Neurol 273:114–125
Shen W, Lee SR, Araujo J et al (2014) Effect of glucocorticoids on neuronal and vascular pathology in a transgenic model of selective Müller cell ablation. Glia. 62(7):1110–1124
Brooks HL, Caballero S, Newell CK et al (2004) Vitreous kevels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol 122(12):1801–1807
Itakura H, Akiyama H, Hagimura N et al (2006) Triamcinolone acetonide suppresses interleukin-1 beta-mediated increase in vascular endothelial growth factor expression in cultured rat Müller cells. Graefes Arch Clin Exp Ophthalmol 244(2):226–231
Shen W, Fruttiger M, Zhu L et al (2012) Conditional Müller cell ablation causes independent neuronal and vascular pathologies in a novel transgenic model. J Neurosci 32(45):15715–15727
Sulaiman RS, Kadmiel M, Cidlowski JA (2018) Glucocorticoid receptor signaling in the eye. Steroids. 133:60–66
Ramamoorthy S, Cidlowski JA (2016) Corticosteroids: mechanisms of action in health and disease. Rheum Dis Clin 42(1):15–31
Ameyar M, Wisniewska M, Weitzman JB (2003) A role for AP-1 in apoptosis: the case for and against. Biochimie. 85(8):747–752
Rogatsky I, Zarember KA, Yamamoto KR (2001) Factor recruitment and TIF2/GRIP1 corepressor activity at a collagenase-3 response element that mediates regulation by phorbol esters and hormones. EMBO J 20(21):6071–6083
Chinenov Y, Gupte R, Dobrovolna J et al (2012) Role of transcriptional coregulator GRIP1 in the anti-inflammatory actions of glucocorticoids. Proc Natl Acad Sci 109(29):11776–11781
Nelson G, Wilde GJ, Spiller DG et al (2003) NF-κB signalling is inhibited by glucocorticoid receptor and STAT6 via distinct mechanisms. J Cell Sci 116(12):2495–2503
Liu T, Zhang L, Joo D et al (2017) NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy 2:17023
Caldenhoven E, Liden J, Wissink S et al (1995) Negative cross-talk between RelA and the glucocorticoid receptor: a possible mechanism for the anti-inflammatory action of glucocorticoids. Mol Endocrinol 9(4):401–412
Morikawa M, Derynck R, Miyazono K (2016) TGF-β and the TGF-β family: context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol 8(5):a021873
Inman GJ (2005) Linking Smads and transcriptional activation. Biochem J 386(1):E1
Yafai Y, Iandiev I, Lange J et al (2014) Müller glial cells inhibit proliferation of retinal endothelial cells via TGF-β2 and Smad signaling. Glia. 62(9):1476–1485
Gerhardinger C, Dagher Z, Sebastiani P et al (2009) The transforming growth factor-β pathway is a common target of drugs that prevent experimental diabetic retinopathy. Diabetes. 58(7):1659–1667
Song CZ, Tian X, Gelehrter TD (1999) Glucocorticoid receptor inhibits transforming growth factor-β signaling by directly targeting the transcriptional activation function of Smad3. Proc Natl Acad Sci 96(21):11776–11781
Hillmer EJ, Zhang H, Li HS et al (2016) STAT3 signaling in immunity. Cytokine Growth Factor Rev 31:1–15
Yun JH, Park SW, Kim KJ et al (2017) Endothelial STAT3 activation increases vascular leakage through downregulating tight junction proteins: implications for diabetic retinopathy. J Cell Physiol 232(5):1123–1134
Langlais D, Couture C, Balsalobre A et al (2012) The Stat3/GR interaction code: predictive value of direct/indirect DNA recruitment for transcription outcome. Mol Cell 47(1):38–49
Macosko EZ, Basu A, Satija R et al (2015) Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 161(5):1202–1214
Peng YR, Shekhar K, Yan W et al (2019) Molecular classification and comparative taxonomics of foveal and peripheral cells in primate retina. Cell. 176(5):1222–1237
Mages K, Grassmann F, Jägle H et al (2019) The agonistic TSPO ligand XBD173 attenuates the glial response thereby protecting inner retinal neurons in a murine model of retinal ischemia. J Neuroinflammation 16(1):43
Das A, Stroud S, Mehta A et al (2015) New treatments for diabetic retinopathy. Diabetes Obes Metab 17(3):219–230
Fong DS, Girach A, Boney A (2007) Visual side effects of successful scatter laser photocoagulation surgery for proliferative diabetic retinopathy: a literature review. Retina. 27(7):816–824
Dugel PU, Bandello F, Loewenstein A (2015) Dexamethasone intravitreal implant in the treatment of diabetic macular edema. Clin Ophthalmol (Auckland, NZ) 9:1321
Quaggin SE (2012) Turning a blind eye to anti-VEGF toxicities. J Clin Invest 122(11):3849–3851
Van Wijngaarden P, Coster DJ, Williams KA (2005) Inhibitors of ocular neovascularization: promises and potential problems. JAMA. 293(12):1509–1513
Bainbridge JW, Smith AJ, Barker SS et al (2008) Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med 358(21):2231–2239
Le Meur G, Lebranchu P, Billaud F et al (2018) Safety and long-term efficacy of AAV4 gene therapy in patients with RPE65 Leber congenital amaurosis. Mol Ther 26(1):256–268
Wang JH, Ling D, Tu L et al (2017) Gene therapy for diabetic retinopathy: are we ready to make the leap from bench to bedside? Pharmacol Ther 173:1–18
Ideno J, Mizukami H, Kakehashi A et al (2007) Prevention of diabetic retinopathy by intraocular soluble flt-1 gene transfer in a spontaneously diabetic rat model. Int J Mol Med 19(1):75–79
Pechan P, Rubin H, Lukason M et al (2009) Novel anti-VEGF chimeric molecules delivered by AAV vectors for inhibition of retinal neovascularization. Gene Ther 16(1):10
Jiang J, Xia XB, Xu HZ et al (2009) Inhibition of retinal neovascularization by gene transfer of small interfering RNA targeting HIF-1α and VEGF. J Cell Physiol 218(1):66–74
Haurigot V, Villacampa P, Ribera A et al (2012) Long-term retinal PEDF overexpression prevents neovascularization in a murine adult model of retinopathy. PLoS One 7(7):e41511
Shyong MP, Lee FL, Kuo PC et al (2007) Reduction of experimental diabetic vascular leakage by delivery of angiostatin with a recombinant adeno-associated virus vector. Mol Vis 13:133
Gong Y, Chang ZP, Ren RT et al (2012) Protective effects of adeno-associated virus mediated brain-derived neurotrophic factor expression on retinal ganglion cells in diabetic rats. Cell Mol Neurobiol 32(3):467–475
Ramírez M, Wu Z, Moreno-Carranza B et al (2011) Vasoinhibin gene transfer by adenoassociated virus type 2 protects against VEGF-and diabetes-induced retinal vasopermeability. Invest Ophthalmol Vis Sci 52(12):8944–8950
Goswami R, Subramanian G, Silayeva L et al (2019) Gene therapy leaves a vicious cycle. Front Oncol 9:297
Acknowledgments
We thank Gabriele Jäger for her excellent technical assistance.
Funding
The project was funded by the University of British Columbia (UBC) to F.G. by providing financial support via the “UBC-Germany Scholarship” and the German Research Foundation (DFG) to A.G. (GR 4403/2-1) and to S.M.H. (HA6014/5-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghaseminejad, F., Kaplan, L., Pfaller, A.M. et al. The role of Müller cell glucocorticoid signaling in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 258, 221–230 (2020). https://doi.org/10.1007/s00417-019-04521-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04521-w